Indium (III) bromide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ In 3+ __ Br - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Indium (III) bromide | |||||||||||||||
| other names |
Indium tribromide |
|||||||||||||||
| Ratio formula | InBr 3 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 354.53 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.96 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
436 ° C |
|||||||||||||||
| boiling point |
656 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Indium (III) bromide is an inorganic chemical compound of indium from the group of bromides .
Extraction and presentation
Indium (III) bromide can be obtained by reacting indium with bromine vapor .
Aqueous solutions of indium (III) bromide can easily be obtained from indium and hydrobromic acid . When concentrated above 33 ° C, anhydrous indium (III) bromide separates from these. Below 33 ° C, hydrates exist as soil bodies.
properties
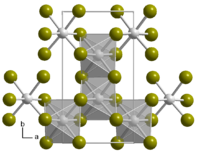
Indium (III) bromide is a light gray solid that is soluble in water and ethanol . It begins to sublime at 371 ° C. The compound crystallizes monoclinically , space group C 2 / m (space group no. 12) with the lattice parameters a = 6.646 Å , b = 11.64 Å, c = 6.633 Å and β = 109.0 °. It consists of layers of InBr 6 - octahedra which are joined at three edges.
use
Indium (III) bromide is a versatile water-tolerant Lewis acid - catalyst . Examples of use include the dithioacetalization of aldehydes in non-aqueous and aqueous media, the addition of trimethylsilyl cyanide to ɑ-hetero-substituted ketones , the conversion of oxiranes to thiiranes with potassium thiocyanate and the addition of indoles to enones and to chiral epoxides .
Individual evidence
- ↑ a b c d e f Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 867.
- ↑ a b c d e f g data sheet Indium (III) bromide, 99.999% trace metals basis from Sigma-Aldrich , accessed on March 17, 2014 ( PDF ).
- ↑ T. Staffel, G. Meyer: In 5 Br 7 , the second mixed-valence In (I) -In (II) bromide: In (I) 3 (In (II) 2 Br 6 ) Br. In: Journal for inorganic and general chemistry , 563, 1988, pp. 27-37, doi: 10.1002 / zaac.19885630105 .
- ^ William W. Porterfield: Inorganic Chemistry . Academic Press, 2013, ISBN 0-323-13894-2 , pp. 132 ( limited preview in Google Book search).
- ↑ Tetrahedron Lett., 41 , 9695 (2000).
- ↑ Tetrahedron Lett., 42 , 3041 (2001).
- ^ Synlett, 396 (2003)
- ↑ J. Org. Chem., 67 , 3700 (2002); 68 , 7126 (2003)
- ↑ J. Org. Chem., 67 , 5386 (2002).

