Trimethylsilyl cyanide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
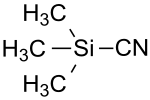
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trimethylsilyl cyanide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 9 NSi | |||||||||||||||
| Brief description |
clear, colorless to yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 99.21 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
|
|||||||||||||||
| Vapor pressure |
approx. 51 hPa at 20 ° C |
|||||||||||||||
| solubility |
Rapid decomposition in water and protic solvents, soluble in organic solvents such as dichloromethane and chloroform |
|||||||||||||||
| Refractive index |
1.392 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Trimethylsilyl cyanide ( TMSCN ) is an activated form of hydrogen cyanide in which the hydrogen atom has been replaced by a trimethylsilyl group . In cyanosilylation with TMSCN, i. H. 1,2 addition of a cyano and trimethylsilyl group to a double bond , such as. B. to the C = O bond of aldehydes and ketones , cyanohydrins - also optically active - are formed in excellent yields. Trimethylsilylnitrile is also a valuable synthon for the preparation of isonitriles , α-aminonitriles, β-hydroxynitriles, 2-cyanopyridines, etc. in very good yields. TMSCN quickly forms highly toxic hydrocyanic acid with humidity .
Manufacturing
The production of trimethylsilyl cyanide is formally based on the reaction of a trimethylsilyl compound TMS- X , such as chlorotrimethylsilane TMS-Cl with hydrogen cyanide HCN or metal cyanides Y -CN, such as potassium cyanide, with the formation of a metal halide YX that is sparingly soluble in the organic medium .
Older methods of making trimethylsilyl cyanide use expensive reagents, such as. B. silver cyanide , the difficult to handle gaseous hydrogen cyanide or large excesses of alkali metal cyanides with phase transfer catalysts and require relatively long reaction times to achieve only modest yields <70%. The procedure from Organic Syntheses with lithium cyanide (from acetone cyanohydrin and lithium hydride ) and TMS-Cl also appears to be complex, cumbersome and unproductive with yields between 59 and 82%.
A solvent-free and catalyst-free variant uses an equimolar mixture of trimethylsilyl chloride and hexamethyldisilazane , which reacts with excess hydrogen cyanide within 1.5 hours at room temperature. After filtering off the by-product ammonium chloride and distillation, TMSCN is obtained in a 98% pure yield.
A variant comes from Manfred T. Reetz's working group in which trimethylsilyl chloride is reacted with equimolar amounts of an alkali metal cyanide in the presence of catalytic amounts of potassium iodide KI and N-methylpyrrolidone NMP at room temperature.
The yields of 87 to 90% are significantly higher than in the older processes with alkali metal cyanides, but the reaction time of approx. 12 hours for one-molar batches is still too high for a technical process.
A semi-continuous process variant appears technically more efficient, in which TMS-Cl is reacted with an approximately equimolar amount of alkali metal cyanide in the presence of catalytic amounts of copper (I) cyanide in sulfolane as a solvent at approx. 180 ° C, the resulting TMSCN with non- converted TMS-Cl distilled off. The mixture is separated by distillation and the TMS-Cl is returned to the reactor. The added TMS-Cl is fully implemented within 2 hours.
When more TMS-Cl and NaCN are metered into the sulfolane / CuCN reaction mixture, the reaction can proceed again, a total yield of trimethylsilyl cyanide of 94% being achieved.
properties
Trimethylsilyl cyanide is a clear, colorless to yellowish, thermally stable, but flammable liquid that has a pungent smell of hydrocyanic acid and is in water and protic solvents, such as. B. methanol rapidly decomposes with the formation of very toxic hydrogen cyanide. TMSCN is soluble in aprotic organic solvents such as dichloromethane or chloroform.
Applications
The reaction of carboxylic acid chlorides with trimethylsilyl cyanide leads to the corresponding acyl cyanides in smooth reaction with high yields.
Pivaloyl cyanide and benzoyl cyanide as starting compounds for the herbicides metribuzin and metamitron are easily accessible in this way.
In tert- alkyl halides, which react with alkali metal cyanides mostly with elimination , the halide is exchanged for the nitrile group with trimethylsilyl cyanide in the presence of tin (IV) chloride in useful yields (> 70%).
In the Reissert-Henze reaction , cyanrimethylsilane reacts with pyridine N-oxide in the presence of triethylamine in acetonitrile to form 2-cyanopyridine in 80% yield .
Stressed ring connections, such as. B. Oxiranes , oxetanes or aziridines react with TMSCN in the presence of catalytic amounts of potassium cyanide and 18-crown-6 with ring opening , the cyanide ion always being added to the least substituted carbon atom.
The ambident character of the cyanide anion is evident in the catalysis of reactions with trimethylsilyl cyanide with soft Lewis acids , such as zinc iodide ZnI 2 , or tin (II) chloride SnCl 2 , whereby β-hydroxyisonitriles are formed from epoxides , the useful precursors for β- amino alcohols and represent oxazolines . In the case of the epoxide cyclohexene oxide , cyanosilylation in the presence of zinc iodide produces the TMS-protected cyanohydrin, from which the protective group can be split off practically quantitatively with potassium fluoride KF and the end product trans-2-isocyanocyclohexanol can be isolated in 76% overall yield.
In a one-pot reaction in water at room temperature, equimolar amounts of aldehydes and amines react with trimethylsilyl nitrile in the presence of indium powder in very good yields (up to 98%) in a Strecker synthesis to form the corresponding α-amino nitriles, from which α- Amino acids are obtained.
Trimethylsilyl cyanide is the standard reagent for cyanosilylation, the conversion of carbonyl compounds such as aldehydes and ketones to achiral and chiral cyanohydrins (α-hydroxynitriles). As the research group led by George A. Olah was able to show, the reaction in dimethylformamide DMF does not require any catalysts, but is achieved by adding carbonates , such as. B. potassium carbonate K 2 CO 3 and phosphates , such as. B. Potassium phosphate K 3 PO 4 accelerated significantly.
The yields achieved are usually well over 80%.
The cyanosilylation of the less reactive ketones requires the addition of catalysts, such as. B. zinc iodide , the benzophenone cyanohydrin being obtained in 79 to 86% yield from the silylated cyanohydrin after hydrolysis with dilute hydrochloric acid .
Sensitive ketones such as 2-acetylfuran can also be converted into achiral cyanohydrin with cyanotrimethylsilane in the presence of N-methylmorpholine-N-oxide in high yield (91%).
A method of enantioselective synthesis for the preparation of chiral cyanohydrins by means of chiral boron compounds using TMSCN and triphenylphosphine oxide with high yields and enantiomeric excesses > 90% ee originates from the working group of Elias James Corey .
Individual evidence
- ↑ a b c Entry on trimethylsilyl cyanide in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b c d e data sheet trimethylsilyl cyanide 98% from Sigma-Aldrich , accessed on June 25, 2018 ( PDF ).
- ↑ a b c data sheet Trimethylsilyl cyanide, 98% from AlfaAesar, accessed on June 25, 2018 ( PDF )(JavaScript required) .
- ↑ Data sheet trimethylsilyl cyanide for synthesis (PDF) from Merck , accessed on June 25, 2018.
- ↑ a b c W.C. Groutas, Z. Jin, H. Zhang: Cyanotrimethylsilane . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2011, doi : 10.1002 / 047084289X.rc276.pub2 .
- ↑ a b T. Livinghouse: trimethylsilyl cyanide: Cyanosilation of p-benzoquinone in: Organic Syntheses . 60, 1981, p. 126, doi : 10.15227 / orgsyn.060.0126 ; Coll. Vol. 7, 1990, p. 517 ( PDF ).
- ↑ E. Soleimani: Trimethylsilyl Cyanide (TMSCN) . In: Synlett . tape 10 , 2017, p. 1625-1626 , doi : 10.1055 / s-2007-982537 .
- ↑ Patent US5258534 : Preparation of trimethylsilyl nitrile. Filed February 5, 1993 , published November 2, 1993 , applicant: Huls America, Inc., inventor: GL Larson, TV John, RR Chawla, CS Subramaniam.
- ↑ Patent US4429145 : Preparation of trimethylsilyl cyanide. Registered on September 14, 1982 , published on January 31, 1984 , applicant: Bayer AG, inventor: MT Reetz, I. Chatziiosifidis.
- ↑ a b Patent EP0040356A2 : Process for the production of trimethylsilyl cyanide. Registered on May 16, 1980 , published on November 25, 1981 , applicant: Bayer AG, inventor: K. Findeisen, K.-H. Left.
- ↑ R. Sustmann: Comprehensive Organic Synthesis, Volume 6, hetero atom manipulation, 1st Edition . Pergamon Press, Oxford 1991, ISBN 0-08-040597-5 , pp. 317-318 .
- ^ MT Reetz, I. Chatziiosifidis, H. Künzer, H. Müller-Starke: Trimethylsilyl cyanide promoted cyanation of tertiary alkyl chlorides and other SN1 active compounds . In: Tetrahedron . tape 39 , no. 6 , 1983, pp. 961-965 , doi : 10.1016 / S0040-4020 (01) 88594-X .
- ↑ H. Vorbrüggen, K. Krolikiewicz: Trimethylsilanol as Leaving Group; III1. A Simple One-Step Conversion of Aromatic Heterocyclic N-Oxides to α-Cyano Aromatic N-Heterocycles . In: Synthesis . tape 4 , 1983, p. 316-319 , doi : 10.1055 / s-1983-30321 .
- ↑ MB Sassaman, GKSurya Prakash, GA Olah: Synthetic methods and reactions. 144. Regiospecific and chemoselective ring opening of epoxides with trimethylsilyl cyanide-potassium cyanide / 18-crown-6 complex . In: J. Org. Chem. Band 55 , no. 7 , 1990, pp. 2016–2018 , doi : 10.1021 / jo00294a012 .
- ↑ PG Gassman, TL Guggenheim: Conversion of epoxides to β-hydroxy isocyanides In: Organic Syntheses . 64, 1986, p. 39, doi : 10.15227 / orgsyn.064.0039 ; Coll. Vol. 7, 1990, p. 294 ( PDF ).
- ↑ D. Bandyopadhyay, JM Velazquez, BK Banik: A truly green synthesis of α-aminonitriles via Strecker reaction . In: Org. Med. Chem. Lett. tape 1 , 2011, p. 1-11 , doi : 10.1186 / 2191-2858-1-11 .
- ↑ GK Surya Prakash, H. Vaghoo, C. Panja, V. Surampudi, R. Kultyshev, T. Mathew, GA Olah: Effect of carbonates / phosphates as nucleophilic catalysts in dimethylformamide for efficient cyanosilylation of aldehydes and ketones . In: PNAS . tape 104 , no. 9 , 2007, p. 3026-3030 , doi : 10.1073 / pnas.0611309104 .
- ↑ PG Gassmann, JJ Talley: Conversion of ketones to cyanohydrins: Benzophenone cyanohydrin In: Organic Syntheses . 60, 1981, p. 14, doi : 10.15227 / orgsyn.060.0014 ; Coll. Vol. 7, 1990, p. 20 ( PDF ).
- ↑ SS Kim, DW Kim, G. Rajagopal: Mild and efficient silylcyanation of ketones catalyzed by N-methylmorpholine-N-oxide . In: Synthesis . tape 2 , 2004, p. 213-216 , doi : 10.1055 / s-2003-44380 .
- ↑ DH Ryu, EJ Corey: Highly enantioselective cyanosilylation of aldehydes catalyzed by a chiral oxazaborolidinium ion . In: J. Am. Chem. Soc. tape 126 , no. 26 , 2004, p. 8107-8107 , doi : 10.1021 / ja0475959 .










