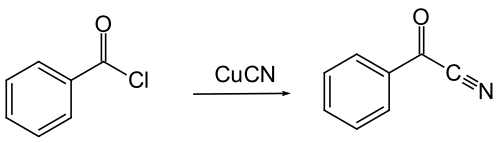
Benzoyl cyanide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Benzoyl cyanide | |||||||||||||||
| other names |
Phenylglyoxylonitrile |
|||||||||||||||
| Molecular formula | C 8 H 5 NO | |||||||||||||||
| Brief description |
colorless to yellowish solid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 131.13 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.11 g cm −3 |
|||||||||||||||
| Melting point |
30-32 ° C |
|||||||||||||||
| boiling point |
206 ° C |
|||||||||||||||
| Vapor pressure |
1.9 mbar (50 ° C) |
|||||||||||||||
| solubility |
decomposes in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Benzoyl cyanide is a chemical compound from the group of nitriles . Analogously to the carboxylic acid halides , the compound can be used as a carboxylic acid cyanide , i.e. H. can be understood as the cyanide of benzoic acid .
Occurrence
Benzoyl cyanide occurs naturally as a poison from some bipeds .
Extraction and presentation
Benzoyl cyanide can be obtained by reacting benzoyl chloride with copper (I) cyanide .
properties
Benzoyl cyanide is a colorless to yellowish solid with a pungent odor that decomposes in water to form phenylglyoxylic acid ( benzoylformic acid ) .
use
Benzoyl cyanide is used as an intermediate in the manufacture of other chemical compounds (e.g. crop protection products ). It serves as a reagent for selective acylation of amino compounds.
safety instructions
The vapors of benzoyl cyanide can form an explosive mixture with air ( flash point approx. 74 ° C, ignition temperature approx. 500 ° C).
Individual evidence
- ↑ a b c d e f g h i j k Entry on benzoyl cyanide in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b Datasheet Benzoyl cyanide, 98% from Sigma-Aldrich , accessed on March 9, 2013 ( PDF ).
- ↑ Thomas Eisner, Jerrold Meinwald, National Academy of Sciences (US): Chemical ecology: the chemistry of biotic interaction . National Academies, 1995, pp. 30 ( limited preview in Google Book search).
- ↑ TS Oakwood, CA Weisgerber: Benzoyl Cyanide In: Organic Syntheses . 24, 1944, p. 14, doi : 10.15227 / orgsyn.024.0014 ; Coll. Vol. 3, 1955, p. 112 ( PDF ).