Reissert reaction
The Reissert reaction is a name reaction in organic chemistry that enables the functionalization of six-membered aromatic nitrogen compounds , generally pyridine or benzene-fused heterocycles such as quinoline and isoquinoline .
Overview reaction
The Reissert reaction can be summarized with the following reaction scheme:
In this reaction, quinoline is converted with a carboxylic acid chloride, here colored blue , and a cyanide nucleophile, colored green , to form the Reissert component.
Reaction mechanism
The initial activation of the aromatic is usually achieved by reacting the aromatic base with an acid chloride (both carboxylic acid chlorides and sulfonic acid chlorides ). The acylation of the aromatic nitrogen atom increases the electrophilicity of the system so that the ammonium ion formed (especially pyridinium, quinolinium or isoquinolinium) can be attacked by the cyanide nucleophile.
This nucleophilic attack leads to the dearomatization of the system with the formation of a diene structure. If a sulfonic acid chloride is used as the activating reagent, the aromatic character of the system can be restored through intramolecular redox processes under the action of a non-nucleophilic base (e.g. DBU ) . The sulfonyl group ( Ts in the example shown ) is reduced to the corresponding sulfinic acid (TsH corresponds to TolSO 2 H, para-toluenesulfinic acid) and a derivative functionalized with a cyano group is obtained as the product . When using carboxylic acid chlorides, the cyanated N- acyl derivative can be deprotonated with strong bases (e.g. NaH ) and converted into many other derivatives by alkylation and subsequent alkaline hydrolysis. The acidification of the methine function by the nitrile group is used here.
application
The Reissert reaction is a key step in the synthesis of isoquinoline derivatives such as the worm control praziquantel .
Reissert-Henze reaction
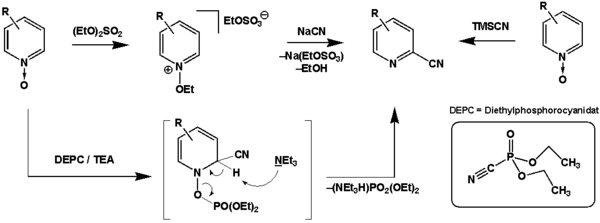
Just like the Reissert reaction itself, the Reissert-Henze reaction (also known as the Reissert-Kaufmann reaction ) enables the synthesis of heterocyclic cyanoaromatics. In contrast to the Reissert reaction, however, N -oxides serve as the starting material. The Reissert reaction itself is mostly unsuccessful on pyridine- N- oxides, but there are exceptions (e.g. 4-chloropyridine- N -oxide). Similar to the Boekelheide rearrangement , the rearomatization is achieved by breaking the NO bond. To do this, the N -oxide function must first be activated. In the classic protocol, alkylation with dialkyl sulfates (see dimethyl sulfate ) is used for this purpose , but there are also modifications that enable activation by silylation (see trimethylsilyl cyanide ) or phosphorylation (see diethyl phosphorocyanidate ).
Individual evidence
- ↑ Thomas L. Gilchrist: Heterocyclic Chemistry. 3. Edition. 1998, ISBN 0-582-27843-0 , p. 169.
- ↑ JA Joule, K. Mills: Heterocyclic Chemistry. 4th edition. 2004, ISBN 0-632-05453-0 , p. 131 f.
- ^ RA Abramovitch, Erwin Klingsberg: Pyridine and its Derivatives. Supplement, Part 2. (Part 2, Volume 14), ISBN 0-471-37914-X , p. 117. ( The Chemistry of Heterocyclic Compounds. V. 14).
- ^ RA Abramovitch, Erwin Klingsberg: Pyridine and its Derivatives. Supplement, Part 2. (Part 2, Volume 14), ISBN 0-471-37914-X , p. 114. ( The Chemistry of Heterocyclic Compounds. V. 14).
- ^ Jie Jack Li: Name Reactions in Heterocyclic Chemistry. 1st edition. 2005, ISBN 0-471-30215-5 , p. 345 ff.