Thallium (I) iodide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
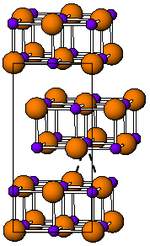
| __ Tl + __ I - | ||||||||||||||||
| Crystal system |
orthorhombic |
|||||||||||||||
| Space group |
Cmcm (No. 63) |
|||||||||||||||
| Lattice parameters |
a = 458.2 pm, b = 1292 pm, c = 525.1 pm |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Thallium (I) iodide | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | TlI | |||||||||||||||
| Brief description |
yellow solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 331.29 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
7.29 g cm −3 |
|||||||||||||||
| Melting point |
440 ° C |
|||||||||||||||
| boiling point |
824 ° C |
|||||||||||||||
| solubility |
practically insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−124 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Thallium (I) iodide is a chemical compound from the group of thallium compounds and iodides .
Extraction and presentation
Thallium (I) iodide can be obtained by reacting thallium (I) sulfate or thallium (I) nitrate with potassium iodide .
properties
Thallium (I) iodide occurs in two enantiotropic modifications. Below 168 ° C it has an orthorhombic crystal structure with the space group Cmcm (space group no. 63) (a = 458.2, b = 1292, c = 525.1 pm). It changes color when exposed to light. At temperatures above 168 ° C it is present as a red solid with a crystal structure of the cesium chloride type. This structure remains unchanged for some time even after cooling to room temperature.
use
Thallium (I) iodide is used as a mixed crystal together with thallium (I) bromide as thallium bromide iodide in the attenuated total reflection spectroscopy .
Individual evidence
- ↑ a b c d e f g Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 876.
- ↑ a b Data sheet Thallium (I) iodide from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry thallium compounds, with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA ), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ AF Holleman , N. Wiberg : Inorganische Chemie . 103rd edition. Volume 1: Basics and main group elements. Walter de Gruyter, Berlin / Boston 2016, ISBN 978-3-11-049585-0 , p. 1393 (reading sample: Part A - Basics of the chemistry of hydrogen. Google book search ).
- ↑ J Michael Hollas: Modern methods in spectroscopy ; ISBN 978-3-540-67008-7 , p. 59.



