Thallium bromide iodide
| Crystal structure | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
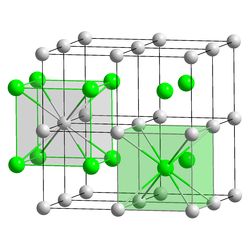
| __ Tl + __ Br - and I - | |||||||||
| Crystal system |
cubic |
||||||||
| Space group |
Pm 3 m (No. 221) |
||||||||
| Coordination numbers |
Cs [8], Br and I [8] |
||||||||
| General | |||||||||
| Surname | Thallium bromide iodide | ||||||||
| other names |
|
||||||||
| Ratio formula | Tl (Br, I) | ||||||||
| Brief description |
red, odorless solid |
||||||||
| External identifiers / databases | |||||||||
|
|||||||||
| properties | |||||||||
| Molar mass | 311.53 g mol −1 (mean value at 42% TlBr and 58% TlI) |
||||||||
| Physical state |
firmly |
||||||||
| density |
7.37 g cm −3 |
||||||||
| Melting point |
414.5 ° C |
||||||||
| solubility |
|
||||||||
| safety instructions | |||||||||
|
|||||||||
| MAK |
0.1 mg m −3 |
||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Thallium bromide iodide Tl (Br, I), also known under the code name KRS-5 used in industry , is an inorganic- chemical mixed crystal compound . It can be seen as a mixed compound of thallium (I) bromide and thallium (I) iodide . Due to its extraordinary optical properties, this compound is of particular importance in infrared spectroscopy .
properties
Industrially used thallium bromide iodide is typically formally composed of 42% thallium (I) bromide and 58% thallium (I) iodide and accordingly has an average molar mass of 311.53 g / mol. In the crystal structure , thallium (Tl + ) takes up 100% of the cation positions, while the anion positions are statistically 42% occupied by bromide (Br - ) and 58% iodide (I - ), so it is one Mixed crystal .
The red salt crystallizes in the cubic crystal system in the space group Pm 3 m (space group no. 221) and is isotypic to the cesium chloride structure . The crystals are very soft, they have a hardness of only 40 K according to Knoop (comparison: diamond has 7000 K). Similar to potassium bromide , it is a cold-flowing material and therefore easily malleable.
Thallium bromide iodide has a high refractive index , which is between 2.73 (at 0.5 µm) and 2.15 (at 50 µm) depending on the wavelength. This results in relatively low losses during reflection (cf. Fresnel's equations ); the maximum transmittance of a 1 mm thick KRS-5 window in the infrared range is around 72% (2 reflections). If you compare this with other materials such as ZnSe , KBr or AMTIR , it is noticeable that thallium bromide iodide has an approximately consistently high degree of transmission over a large wavelength range (2-50 µm). For comparison, quartz glass has a higher degree of transmission, but only in smaller wavelength ranges (90% at 0.3–2 µm; 80% at 100–600 µm). Diamond, on the other hand, shows a large area with a high degree of transmission (up to 65%), but diamond is much more expensive and more difficult to process.
It only dissolves to about 0.05% in water. In contrast, it is soluble in some polar, organic solvents, such as ethylene glycol or dimethyl sulfoxide (DMSO) . In addition, it can dissolve in an alkaline aqueous medium and in the presence of complexing agents .
use
The good permeability for infrared light makes thallium bromide iodide an ideal material for the production of windows, lenses and filters for optical devices that work in the infrared range, for example infrared cameras or infrared spectrometers . The high refractive index enables another application in the infrared spectroscopic area , ATR spectroscopy , in which the attenuated total reflection is used to investigate surface properties.
hazards
As a thallium compound, thallium bromide iodide is considered to be potentially toxic. Its low solubility in water, due to its thallium (I) iodide content, alone makes it safer to use in the field of spectroscopy. Special care should be taken, however, as thallium bromoiodide dissolves in some organic solvents. Dissolved thallium salts are extremely toxic.
Web links
- LOT-Oriel-Lasers data sheet ( Memento from December 7th, 2008 in the Internet Archive ) (PDF file; 86 kB)
- Physical data at Korth Kristalle GmbH
Individual evidence
- ↑ a b c Safety data sheet TlCl, TlBr, KRS-5, KRS-6, crystalline (PDF; 121 kB) from Korth Kristalle GmbH, accessed April 18, 2013.
- ↑ a b c Physical data KRS-5 (Tl (Br-J)) from Korth Kristalle GmbH, accessed April 18, 2013.
- ↑ a b Data sheet Thallium (I) bromide from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry thallium compounds, with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA ), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thallium Bromoiodide (KRS-5) Optical Crystals. International Crystal Laboratories, accessed May 4, 2010 .


