Thallium (I) nitrate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
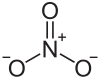
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Thallium (I) nitrate | |||||||||||||||
| other names |
Thallium nitrate (ambiguous) |
|||||||||||||||
| Molecular formula | TlNO 3 | |||||||||||||||
| Brief description |
moisture-sensitive, hygroscopic white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 266.39 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
5.55 g cm −3 |
|||||||||||||||
| Melting point |
206 ° C |
|||||||||||||||
| boiling point |
430 ° C |
|||||||||||||||
| solubility |
soluble in water (95 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Thallium (I) nitrate is a chemical compound from the group of thallium compounds and nitrates .
Extraction and presentation
Thallium (I) nitrate can be obtained by reacting thallium (I) iodide with nitric acid. However, it is easier to manufacture starting from the metal itself, its hydroxide or the carbonate:
properties
Thallium (I) nitrate is a moisture-sensitive, hygroscopic, white, odorless, oxidizing solid. It decomposes when heated, producing nitrous gases and thallium oxides. It is soluble in water, the solubility increasing extremely strongly when heated.
use
Thallium (I) nitrate is used for chemical production and analysis and as an additive in the production of fiber optic lenses .
Related links
Web links
- EPA: Toxicological Review of Thallium nitrate (PDF; 1.1 MB)
Individual evidence
- ↑ a b c d e f g Entry on thallium (I) nitrate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b Teck: MSDS Thallium (I) nitrate ( Memento from July 5, 2011 in the Internet Archive )
- ↑ a b data sheet thallium (I) nitrate from Sigma-Aldrich , accessed on December 16, 2010 ( PDF ).
- ^ R. Pribil, V. Veselý, K. Kratochvíl: Contributions to the basic problems of complexometry - IV: Determination of thallium . In: Talanta . tape 8 , no. 1 , 1961, pp. 52-54 , doi : 10.1016 / 0039-9140 (61) 80037-4 .
- ^ Heinrich Remy: Textbook of Inorganic Chemistry Volume I + II, Leipzig 1973.
- ^ Thallium ( Memento from March 6, 2013 in the Internet Archive )





