Thallium (I) fluoride
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ Tl + __ F - | ||||||||||||||||
| Crystal system |
orthorhombic |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Thallium (I) fluoride | |||||||||||||||
| other names |
|
|||||||||||||||
| Ratio formula | TlF | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 223.39 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
8.36 g cm −3 |
|||||||||||||||
| Melting point |
327 ° C |
|||||||||||||||
| boiling point |
655 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Thallium (I) fluoride is a chemical compound from the group of thallium compounds and fluorides .
Extraction and presentation
Thallium (I) fluoride can be obtained by reacting thallium (I) carbonate with pure hydrogen fluoride .
properties
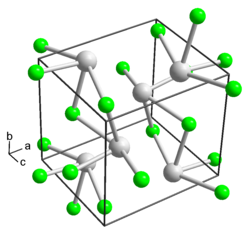
Thallium (I) fluoride is in the form of hard, shiny white crystals with an orthorhombic crystal structure (deformed rock salt structure , a = 518.48, b = 609.80, c = 549.16 (2) pm, Z = 4, space group Pm 2 a (room group no. 28, position 6) ). As a liquid it is yellowish. It is not hygroscopic , but dissolves when you breathe on it and immediately solidifies again. The concentrated aqueous solution shows strong alkaline reactions .
use
Thallium (I) fluoride can be used to make fluorine-containing esters .
See also
Individual evidence
- ↑ a b c d e f g h i Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 876.
- ↑ a b Data sheet Thallium (I) fluoride from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry thallium compounds, with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA ), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Crystal structure and lattice energy of thallium (I) fluoride: inert-pair distortions , doi : 10.1039 / DT9740001907 .



