Beryllium sulfate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
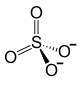
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Beryllium sulfate | ||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| Brief description |
white odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 105.08 g mol −1 (anhydrous) 177.14 g mol −1 (tetrahydrate) |
||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.71 g cm −3 (tetrahydrate) |
||||||||||||||||||
| Melting point |
580 ° C (decomposition) |
||||||||||||||||||
| solubility |
very soluble in water, insoluble in ethanol |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−1205.2 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Beryllium is a white beryllium - salt of sulfuric acid with the formula BeSO 4 . It was first isolated by Berzelius in 1815 .
Manufacturing
Beryllium sulfate can be obtained by dissolving beryllium carbonate or beryllium hydroxide in dilute sulfuric acid.
properties
Physical Properties
There are several hydrates of beryllium sulfate: The compound is commercially available as a tetrahydrate with the empirical formula BeSO 4 · 4 H 2 O, which converts to the dihydrate BeSO 4 · 2 H 2 O at 111.5 ° C and into the dihydrate at 158 ° C Monohydrate BeSO 4 · H 2 O passes over. This can then be dehydrated at approx. 400 ° C to form anhydrous beryllium sulfate. The anhydrous sulfate itself is stable up to approx. 580 ° C. There is also a hexahydrate BeSO 4 · 6 H 2 O, which converts into the dihydrate at approx. 76 ° C. Anhydrous beryllium sulfate is only slowly and poorly soluble in water. In contrast, the tetrahydrate is readily soluble in water.
Chemical properties
The beryllium ion is present in the salt as [Be (H 2 O) 4 ] 2+ , since the coordination number 4 is typical for beryllium ions (main group element). With alkali metal sulfates, it forms cauliflower-like crusts that crystallize poorly and are understood as double salts with the empirical formula Me 2 Be (SO 4 ) 2 · 2 H 2 O, e.g. E.g .: K 2 Be (SO 4 ) 2 · 2 H 2 O.
Biological importance
Beryllium sulfate is extremely toxic. A strong carcinogenic effect could also be observed in rats . In humans, however, only a few cases are known.
poisoning
Various forms of beryllium poisoning are known, most of which originate from the dusts of the substances:
Toxic pneumonia :
- after repeated or infrequent single inhalations with varying latency periods from a few days to several years for no apparent reason
- Symptoms are shortness of breath, cough and fever
- The disease is slow and death can occur after months of infirmity
Beryllium:
- a slowly developing granulomatous reaction of the lung tissue ("sandstorm lung")
use
A mixture of beryllium sulphate and radium sulphate served as a neutron source when nuclear fission was discovered . Beryllium sulfate is also rarely used in homeopathy .
Individual evidence
- ↑ Beryllium sulfate data sheet at AlfaAesar, accessed on March 11, 2010 ( PDF )(JavaScript required) .
- ↑ a b c data sheet Beryllium sulfate tetrahydrate from Sigma-Aldrich , accessed on July 7, 2019 ( PDF ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , p. 903.
- ↑ Susan Budavari (ed.): The Merck Index . 12th edition. Merck & Co., Whitehouse Station, New Jersey, USA 1996, ISBN 0-911910-12-3 .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the indicated labeling it falls under the group entry beryllium compounds with the exception of aluminum beryllium silicates, and with those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-6.
- ↑ Ch. L. Parsons: The Chemistry and Literature of Beryllium. London 1909, p. 33 ff.
- ↑ a b c M. Levi-Malvano: The hydrates of beryllium sulfate. In: Zeitschr. f. anorg. Chem. 1906, 48, pp. 446ff. (Full text)
- ↑ a b Gmelin's Handbook of Inorganic Chemistry. BERYLLIUM, system number 26, eighth edition. Verlag Chemie, Berlin 1930.
- ↑ M. Binnewies: General and Inorganic Chemistry. 1st edition. 2004, ISBN 3-8274-0208-5 , pp. 355 f.
- ↑ Umwelt-online.de: TRGS 910-14: Beryillium and its compounds ( Memento from April 4, 2018 in the Internet Archive )
- ^ W. Forth: General and special toxicology and pharmacology. 6th edition. 1992, ISBN 3-411-15026-2 , p. 781.




