Beryllium carbonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
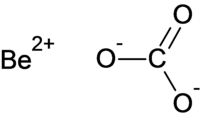
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Beryllium carbonate | |||||||||||||||
| Molecular formula | BeCO 3 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 69.02 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
54 ° C |
|||||||||||||||
| boiling point |
100 ° C (decomposition) |
|||||||||||||||
| solubility |
poorly soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Beryllium carbonate is an inorganic chemical compound of beryllium from the group of carbonates .
Occurrence
Beryllium carbonate occurs naturally in the form of the mineral niveolanite in the form of the mixed compound NaBe (CO 3 ) (OH) · 2H 2 O.
Extraction and presentation
Beryllium carbonate tetrahydrate can be obtained by reacting carbon dioxide with an aqueous solution of beryllium hydroxide. When sodium carbonate is added to a beryllium salt solution and this is reacted with carbon dioxide, beryllium oxide carbonate, which is a mixture of beryllium carbonate and beryllium hydroxide, is formed.
The anhydrate can be obtained by annealing beryllium oxide in a stream of carbon dioxide at 1000 ° C for several hours .
Basic beryllium carbonate Be 2 CO 3 (OH) 2 is formed when beryllium sulfate reacts with ammonium carbonate and contains both carbonate and hydroxide ions.
properties
Beryllium carbonate is a solid that is sparingly soluble in water. It gives off carbon dioxide easily and can only be stored under a CO 2 atmosphere.
Soluble complexes of the compound can be obtained by dissolving beryllium hydroxide in ammonium carbonate solutions.
use
Beryllium carbonate is mainly used as an intermediate in the production of beryllium from beryllium ores.
Individual evidence
- ^ A b c A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1113.
- ↑ a b c d e f Richard C. Ropp: Encyclopedia of the Alkaline Earth Compounds . Newnes, 2012, ISBN 0-444-59553-8 , pp. 359 ( limited preview in Google Book Search).
- ↑ Entry on beryllium compounds in the GESTIS substance database of the IFA , accessed on April 14, 2014(JavaScript required) .
- ↑ Kenneth A. Walsh: Beryllium Chemistry and Processing . ASM International, 2009, ISBN 0-87170-721-7 , pp. 118 ( limited preview in Google Book search).
- ^ Gmelin's Handbook of Inorganic Chemistry. BERYLLIUM, system number 26, eighth edition. Verlag Chemie, Berlin 1930, pages 178-179





