Sodium arsenide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
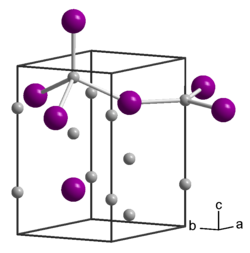
| __ Na + __ As 3− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium arsenide | |||||||||||||||
| other names |
Trisodium arsenide |
|||||||||||||||
| Ratio formula | AsNa 3 | |||||||||||||||
| Brief description |
brown purple solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 143.89 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.36 g cm −3 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium arsenide is an inorganic chemical compound of sodium from the group of arsenides . In addition to Na 3 As, other sodium arsenides such as NaAs are known.
Extraction and presentation
Sodium arsenide can be obtained by reacting sodium vapor with arsenic at 180 to 200 ° C.
properties
Sodium arsenide is a brown-purple solid. It has a hexagonal crystal structure with the space group P 6 3 / mmc (space group No. 194) and the lattice parameters a = 4.874 Å and c = 8.515 Å. The structure contains two crystallographically different Na atoms; one is trigonal-planar surrounded by As atoms, the other tetrahedral . Other literature sources report a structure of the anti- tysonite type with the P 6 3 cm (No. 185) . Arsenic hydrogen is formed on contact with water .
Individual evidence
- ↑ a b c J. D. Smith: The Chemistry of Arsenic, Antimony and Bismuth: Pergamon Texts in Inorganic Chemistry . Elsevier, 2013, ISBN 978-1-4831-8754-9 , pp. 559 ( limited preview in Google Book search).
- ↑ Norbert Auner, Wolfgang A. Herrmann, Uwe Klingebiel: Synthetic Methods of Organometallic and Inorganic Chemistry, Volume 2, 1996: Volume 2: Groups 1,2, 13 and 14 . Georg Thieme Verlag, 2014, ISBN 3-13-179171-3 , p. 40 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ J. Songster, AD Pelton: The As-Na (arsenic-sodium) system. In: Journal of Phase Equilibria. 14, 1993, p. 240, doi : 10.1007 / BF02667819 .
- ↑ William A. Hart, OF Beumel, Thomas P. Whaley: The Chemistry of Lithium, Sodium, Potassium, Rubidium, Cesium and Francium: Pergamon Texts in Inorganic Chemistry . Elsevier, 2013, ISBN 978-1-4831-8757-0 , pp. 439 ( limited preview in Google Book search).
- ↑ HJ Beister, K. Syassen, J. Klein: phase transition of Na 3 pressure As under. In: Journal of Nature Research B . 45, 1990, pp. 1388-1392 ( PDF , free full text).
- ↑ Bodie Douglas, Shi-Ming Ho: Structure and Chemistry of Crystalline Solids: . Springer Science & Business Media, 2007, ISBN 978-0-387-36687-6 , pp. 323 ( limited preview in Google Book search).
- ↑ Amit Arora: Textbook Of Inorganic Chemistry: . Discovery Publishing House, 2005, ISBN 978-81-8356-013-9 , pp. 521 ( limited preview in Google Book search).
- ↑ Peter Hafner, Klaus-Jürgen Range: Na3As revisited: high-pressure synthesis of single crystals and structure refinement. In: Journal of Alloys and Compounds. 216, 1994, p. 7, doi : 10.1016 / 0925-8388 (94) 91033-2 .

