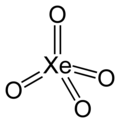
Xenon (VIII) oxide
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Xenon (VIII) oxide | ||||||
| other names |
Xenon tetroxide |
||||||
| Molecular formula | XeO 4 | ||||||
| Brief description |
as a solid: yellow, easily exploding crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 195.29 g mol −1 | ||||||
| Physical state |
gaseous |
||||||
| Melting point |
−39.5 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| Thermodynamic properties | |||||||
| ΔH f 0 | |||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Xenon (VIII) oxide is an oxide of the noble gas xenon and thus a noble gas compound . It is a colorless gas under standard conditions.
Extraction and presentation
Solutions of xenates in the form of HXeO 4 - are used as the starting material for the production of xenon (VIII) oxide . These can be oxidized by disproportionation or with the help of ozone to perxenates of the form HXeO 6 3− and precipitated as barium perxenate Ba 2 XeO 6 . With the help of concentrated sulfuric acid , the unstable intermediate stage perxenonic acid H 4 XeO 6 xenon (VIII) oxide can be obtained:
-
- Formation of the intermediate stage
-
- Formation of xenon (VIII) oxide
These reactions are carried out at −5 ° C, whereby the product xenon tetraoxide escapes as a gas.
properties
Xenon (VIII) oxide has a positive enthalpy of formation of 643 kJ / mol and is therefore a strongly endothermic compound. As a result, it tends to break down explosively with the formation of elemental xenons and molecular oxygen. In the solid state below −39.5 ° C, it is a yellowish, crystalline mass of considerably greater stability. Occasionally it can also explode at low temperatures (−40 ° C), which is why xenon tetraoxide must be handled with great care and at the lowest possible temperatures ( nitrogen cooling ). A sudden explosion is triggered when the heat of reaction accumulated or (oxygen) radicals accelerate the further reaction due to gradual decomposition .
Aqueous solution
In aqueous solution, xenon (VIII) oxide is present in the form of HXeO 6 3− ions, which can be derived from the inconsistent and non-isolable perxenonic acid H 4 XeO 6 . The reason for their instability lies in the decomposition of perxenonic acid to xenonic acid and oxygen . The decomposition takes place slowly in the basic and very quickly in the acidic . On the other hand, a large number of salts that are derived from perxenonic acid and are called perxenates are stable . Examples are barium perxenate Ba 2 XeO 6 or sodium perxenate Na 4 XeO 6
Individual evidence
- ↑ Entry on xenon connections. In: Römpp Online . Georg Thieme Verlag, accessed on July 14, 2014.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ H. Selig, JG Malm, HH Claassen, CL Chernick, JL Huston: Xenon tetroxide - Preparation and Some Properties. In: Science. 143 (1964), p. 1322.

