Noble gases
|
Position in the periodic table
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| group | 18th |
| Main group | 8th |
| period | |
| 1 |
2 He |
| 2 |
10 Ne |
| 3 |
18 ares |
| 4th |
36 kr |
| 5 |
54 Xe |
| 6th |
86 para |
| 7th |
118 above |
The noble gases , also inert gases or inert gases, form a group in the periodic table of the elements that includes seven elements : helium , neon , argon , krypton , xenon , radioactive radon and the artificially generated, also radioactive oganesson . The group is systematically called 8th main group or, according to the more recent classification of the periodic table, group 18 and is shown on the right-hand edge of the periodic table next to the halogens .
The uniform main characteristic of all noble gas atoms is that all their electron shells are either completely occupied with electrons or empty ( noble gas configuration ): There are only completely filled atomic orbitals , which mean that noble gases only enter into chemical reactions under extreme conditions; they do not form molecules with one another either, but are monatomic and gases at room temperature . They owe their group name to this low reactivity , which is based on the precious metals , which are also not very reactive .
Helium is by far the most common noble gas. Argon is the most common on earth; all others are among the rare components of the earth. As gases they are components of the air ; With the exception of helium, which is contained in natural gas , they are only found in very small quantities in the earth's crust . They were discovered - with the exception of the Oganesson, which was only produced in 2006 - in quick succession in the years 1868 (helium) to 1900 (radon). Most noble gases were first isolated by the British chemist William Ramsay .
Noble gases are mainly used as protective gas , e.g. B. in incandescent lamps , they are important as filling gas for gas discharge lamps , in which they glow in the color characteristic of each gas. Despite the low reactivity of the heavier noble gases, in particular xenon, chemical compounds are known. The most important of these is the strong oxidizing agent xenon (II) fluoride .
history
The first indication that air contains an unreactive gas was found by Henry Cavendish in 1783 . He mixed air and oxygen such that the elements contained therein, nitrogen and oxygen with the aid of static electricity completely nitrogen oxides react. An unreactive residue remained. However, he did not realize that it was a new gas - a mixture of argon and other noble gases - and did not continue his experiments.
In 1868, Jules Janssen and Norman Lockyer discovered helium as the first noble gas, independently of one another. The two astronomers - Janssen in India, Lockyer in England - observed the solar spectrum and discovered a previously unknown yellow spectral line at a wavelength of 587.49 nm . The new element was named helium by Edward Frankland after ancient Greek ἥλιος hélios for the sun . The first evidence of helium on Earth succeeded in 1892 Luigi Palmieri by spectral analysis of Vesuvius - Lava .
Cavendish's experiments in studying the air were continued by Lord Rayleigh from 1888 . He noticed that "nitrogen" obtained from the air has a different density than that obtained from the decomposition of ammonia . Rayleigh therefore suspected that there must be an as yet unknown, inert component in the air. Therefore, he and William Ramsay tried to completely remove the nitrogen from an air sample by reacting with magnesium and to isolate this unknown gas. Finally, in 1894, they succeeded in spectroscopically demonstrating a new element which they named argon after the Greek ἀργός argos , "sluggish" .
After the most important properties of helium and argon had been determined, it was found that these gases, in contrast to the other atmospheric gases, are monatomic. This was recognized by the fact that the ratio of the molar heat capacity C p at constant pressure in relation to the heat capacity C V at constant volume for noble gases has a very high value of 1.67 (= C p / C V ), while di- and polyatomic gases Gases have significantly lower values. Then William Ramsay suspected that there must be a whole group of such gases that form a separate group in the periodic table and he began to look for these. In 1898 he and Morris William Travers succeeded in isolating air, neon , krypton and xenon by fractional distillation .
The last naturally occurring noble gases in 1900 by Friedrich Ernst Dorn as radium emanation (exhalation of radium that) Radon discovered and denoted by the symbol Em. This was the isotope 222 Rn. Further radon isotopes were found by Ernest Rutherford and André-Louis Debierne and initially thought to be separate elements. It was only after William Ramsay determined the spectrum and other properties in 1910 that he realized that it was a single element. He initially called this niton (Nt), the name radon has been used since 1934. Oganesson , the last element of the group, could be produced for the first time in 2002-2005 at the United Institute for Nuclear Research in Dubna after several unsuccessful attempts .
Soon after the discovery, attempts were made to synthesize compounds of the noble gases. In 1894, Henri Moissan tried to achieve a reaction of argon with fluorine , but failed. In 1924 A. von Antropoff claimed to have synthesized the first krypton compound in the form of a red stable solid from krypton and chlorine . However, it later turned out that this compound did not contain krypton, but nitrogen monoxide and hydrogen chloride .
With xenon hexafluoroplatinate , Neil Bartlett discovered a xenon compound for the first time in 1962 and thus the first noble gas compound ever. Only a few months after this discovery, in August 1962, the synthesis of xenon (II) fluoride by Rudolf Hoppe and that of xenon (IV) fluoride by a group led by the American chemists C. L. Chernick and H. H. Claassen followed almost simultaneously . Soon after the first crypto connection could be shown that he initially for by A. V. Grosse krypton tetrafluoride held that as However, after further experiments krypton difluoride was identified. In 2000 the first argon compound, the very unstable argon fluorohydride, was synthesized.
Occurrence
| element | Solar system (atoms rel. To Si (Si = 1 · 10 6 )) |
Earth's atmosphere (volume, ppm) |
Earth's crust (mass, ppm) |
|---|---|---|---|
| Hey | 2.21 · 10 9 | 5.24 | 0.008 |
| No | 3.44 · 10 6 | 18.18 | 0.005 |
| Ar | 1.172 · 10 5 | 9340 | 3.5 |
| Kr | 46.8 | 1.14 | 0.0001 |
| Xe | 5.38 | 0.09 | 3 · 10 −5 |
| Marg | 0.06 ... 18 · 10 −19 | 4 · 10 −13 |
Noble gases are mainly found in the earth's atmosphere , but to a lesser extent also in the earth's crust; however, their frequencies are very different. By far the most common is argon, which, with a volume fraction of 0.934% (9340 ppm), makes up a significant proportion of the entire atmosphere. All others are much rarer with proportions below 20 ppm, so they count among the trace gases . Krypton, xenon and radon are among the rarest elements on earth. Helium is also a component of natural gas , of which it can make up up to 16% of the volume.
Due to its low density, a small amount of helium constantly leaves the earth's atmosphere into space and noble gases are constantly being formed on earth, which largely determines their frequencies and isotope ratios. Argon, especially the isotope 40 Ar, is formed by the decay of the potassium isotope 40 K. Helium arises from the alpha decay of heavy elements such as uranium or thorium (alpha particles), xenon from the rare spontaneous decay of uranium. The short-lived radon isotope 222 Rn with a half-life of 3.8 days is the most common and an intermediate product in the decay series of 238 U. Other, even shorter-lived isotopes are also members of the decay series of uranium, thorium or neptunium isotopes. Due to these decay processes, the noble gases can also be found enclosed in rocks. Helium is found in many uranium ores such as uraninite and argon in the basalt of the oceanic crust, and it only gasses out when the surrounding rock melts.
The frequency distribution of the noble gases in the universe can largely be explained by the nucleosynthesis pathways. The heavier a noble gas, the rarer it is. Helium, which is formed both by primordial nucleosynthesis and from hydrogen by stellar nucleosynthesis, is the second most common element after hydrogen. Neon and argon are also among the most common elements in the universe. Krypton and xenon, which are not produced by stellar nucleosynthesis and only form in rare events such as supernovae , are much rarer. Due to their regular structure with an even number of protons, noble gases are more common than many similarly heavy elements according to Harkins' rule .
Extraction
With the exception of a large part of the helium and the radioactive elements, the noble gases are extracted exclusively from the air. They occur as by-products in the production of nitrogen and oxygen in the Linde process . In the main rectification column , in which oxygen and nitrogen are separated, the various noble gases accumulate at different points. However, they can be transferred to their own column and separated there from all other gases. While argon can easily be separated and only needs to be freed from nitrogen and oxygen, the problem with helium and neon, but also with krypton and xenon, is that they first accumulate together and then have to be separated. This can be done via a further rectification column or by different adsorption of the gases on suitable adsorption media.
Helium has been obtained mainly from natural gas since at least 1980 . This helium source was first discovered in the United States , later also used in the Soviet Union, today in a few other countries and plants, for example in Algeria, the yield of which, liquefied at low temperatures, is shipped to Marseille and thus Europe in a 40-foot container . It can be separated from the other components of the natural gas as crude helium either by freezing out all other gases or by permeation on suitable membranes . The helium then has to be freed from residual interfering gases such as nitrogen or hydrogen by pressure swing adsorption , chemical or cryotechnical processes.
Radon cannot be obtained in large quantities due to its short half-life. On a smaller scale, radium serves as a source, radon is created when this element decays and gasses out from a corresponding preparation. Oganesson could be produced as an artificial element in a few atoms by bombarding Californium with calcium atoms.
properties

Physical Properties
All noble gases are monatomic, colorless and odorless gases under normal conditions. They condense and solidify only at very low temperatures, the melting and boiling points being higher, the greater the atomic mass. At 4.224 K (−268.926 ° C), the boiling point of helium is only slightly above absolute zero, the heaviest noble gas, radon, boils at 211.9 K (−61.25 ° C).
The peculiarity of helium is that it is the only element that does not solidify under atmospheric pressure and well above it. Instead, at 2.17 K it changes into a special state of matter, superfluidity . In this, the liquid loses its internal friction and can, for example, crawl over higher vessel walls ( Onnes effect ). Only at pressures above 25.316 bar does helium solidify at 0.775 K. These temperatures and pressures only apply to the frequent isotope 4 He, whereas the rare, second, lighter, stable isotope 3 He has significantly different properties. It only becomes superfluid at temperatures below 2.6 · 10 −3 K. The melting point, boiling point and critical point are also at different temperatures and pressures.
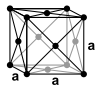
With the exception of helium, which crystallizes in the hexagonal crystal system, all noble gases have a face-centered cubic crystal structure. As can be expected from the increasing atomic radii , the lattice parameter a increases from neon to radon.
As expected, the densities of the noble gases also correlate with the atomic mass. After hydrogen, helium is the gas with the lowest density. As the only other noble gas, neon has a lower density than air, while argon , krypton , xenon and radon are denser. With a density of 9.73 kg / m 3, radon is one of the densest gases of all.
| element | Helium ( 3 He and 4 He) | neon | argon | krypton | xenon | radon | |
|---|---|---|---|---|---|---|---|
| Color gas discharge |  |
 |
 |
 |
 |
red | |
| Melting point (1013 hPa) | 0.319 K (−272.831 ° C) (29.315 bar) |
0.775 K (−272.375 ° C) (25.316 bar) |
24.57 K (−248.58 ° C) |
84.0 K (−189.2 ° C) |
116.2 K (−157.0 ° C) |
161.4 K (−111.8 ° C) |
approx. 202 K (approx. −71 ° C) |
| Boiling point (1013 hPa) | 3.1905 K (−269.9595 ° C) |
4.224 K (−268.926 ° C) |
27.09 K (−246.06 ° C) |
87.295 K (−185.855 ° C) |
119.79 K (−153.36 ° C) |
165.03 K (−108.12 ° C) |
211.9 K (−61.3 ° C) |
| Critical point |
|
|
|
|
|
|
|
| Triple point | unavailable |
|
|
|
|
|
|
| Density (0 ° C, 1013 hPa) | 0.13448 kg / m 3 | 0.17847 kg / m 3 | 0.9000 kg / m 3 | 1.7839 kg / m 3 | 3.7493 kg / m 3 | 5.8971 kg / m 3 | 9.73 kg / m 3 |
| structure | 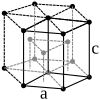 |
 |
 |
 |
 |

|
|
| Crystal system | hexagonal | cubic | cubic | cubic | cubic | cubic | |
| Lattice parameters |
|
a = 4.43 Å |
a = 5.26 Å |
a = 5.72 Å |
a = 6.20 Å |
a = 6.55 Å (calculated) |
|
The properties of Oganesson cannot be determined experimentally due to its short half-life. According to theoretical considerations , due to relativistic effects and the high polarizability of the oganesson atom, it can be assumed that oganesson is significantly more reactive than radon. It is also unlikely to be gaseous under standard conditions ; a boiling point between 320 and 380 K can be assumed by extrapolation.
- Noble gases in gas discharge lamps
Atomic properties
In the case of noble gases, all electron shells are either completely occupied with electrons or empty. This is why this state is also called the noble gas configuration . Helium is the only noble gas in which only one s orbital is fully occupied (since there is no 1p orbital), with all others the outermost occupied orbital is a p orbital . According to the laws of quantum mechanics , this state of the orbitals is particularly favorable in terms of energy. This is why atoms of other elements also tend to reach the noble gas configuration by releasing or accepting electrons ( noble gas rule ).
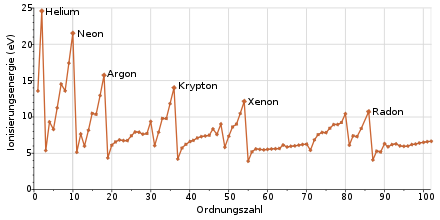
The properties of the noble gases are clearly determined by the fact that they do not achieve the noble gas configuration by releasing or accepting electrons, but rather in a neutral, non-ionized state. Noble gases are therefore monatomic, have a high ionization energy and almost do not react with other elements or compounds.
Chemical properties
Despite the structure of the noble gas atoms, the heavy noble gases are not completely unreactive and can form some compounds. This is due to the greater distance between the valence electrons and the nucleus, which reduces the ionization energy, as well as relativistic effects . The greatest variety of compounds is known from xenon and not, as expected, from radon, since the strong radioactivity and short half-life make the formation of compounds and their investigation more difficult.
The only element that is able to react directly with xenon, radon and, under certain conditions, krypton is fluorine . While the krypton (II) fluoride formed in the reaction of krypton and fluorine is thermodynamically unstable and can therefore only be synthesized at low temperatures, the xenon and radon fluorides are also stable at room temperature. Other elements do not react with noble gases, but various other compounds are known that are accessible through reactions of fluorides.
The reactivity and stability of compounds of the light noble gases helium, neon and argon could only be investigated theoretically, with the exception of one known argon compound, HArF. Accordingly, neon is considered to be the least reactive noble gas. Calculations showed that the neon analogue of the only theoretically stable helium compound, HHeF, should not be stable.
Due to the lack of chemical compounds of the noble gases, there were no numerical values of their electronegativities for a long time - so far only the values of the Pauling scale for the two elements xenon (2.6) and krypton (3.0) could be determined roughly correspond to those of the halogens. In the newer electronegativity scales according to Mulliken , Allred and Rochow, on the other hand, numerical values can also be calculated for the other noble gases, which in this case exceed those of the halogens. For helium, for example, they are 5.50 according to Allred-Rochow and 4.86 according to Mullikan.
use

Noble gases are used in gas discharges due to their low reactivity, low melting points and characteristic colors. Argon and helium in particular are used on a larger scale, the other noble gases can only be produced in smaller quantities and are therefore expensive. The low reactivity is used as an inert or protective gas, for example in gas-shielded welding and in the production of certain metals such as titanium or tantalum . Argon is mainly used for this whenever the cheaper but more reactive nitrogen cannot be used.
In the case of gas discharges, every noble gas emits light of a characteristic color. For example, with neon the emitted light is red, with argon it is violet and with krypton or xenon it is blue. This is used in gas discharge lamps . Xenon is of particular importance, since the spectrum of a xenon gas discharge lamp corresponds approximately to that of daylight . It is therefore also used in car headlights as " xenon light ". Also tubes based on this principle, after the first used coal gas Neon they are also fluorescent lights called. In contrast, the fluorescent lamps known colloquially as "neon tubes" do not use a noble gas, but rather mercury vapor as a light source. Even light bulbs are filled with noble gases, krypton or argon frequently. As a result, the effective evaporation rate of the filament is lower, which enables a higher temperature and thus better light yield.
Because of their low melting and boiling points, noble gases are important as coolants . Above all, liquid helium plays a role here, as it enables particularly low temperatures to be achieved. This is important, for example, for superconducting magnets , which are used in nuclear magnetic resonance spectroscopy . If temperatures as low as those offered by liquid helium do not have to be reached for an application, the higher-boiling noble gases such as neon can also be used.
Like all gases, the noble gases have a narcotic effect, depending on the pressure, by blocking membranes in nerve cells . The pressures required for helium and neon are so high that they can only be achieved in the laboratory; the pressure required for neon is 110 bar. Since they cannot cause a deep intoxication , these two gases are mixed with oxygen (" Heliox " and " Neox "), also with oxygen and nitrogen (" Trimix ") used as breathing gases when diving . With these it is possible to reach greater depths than when using air. Xenon, on the other hand, has a narcotic effect even at ambient pressure and can therefore be used as an inhalation anesthetic instead of nitrous oxide . However, it is rarely used due to its high price and low availability.
Helium is filling and lifting gas for gas balloons and zeppelins . In addition to helium, hydrogen can also be used. This is lighter and allows more payload, but it can react with the oxygen in the air and burn. This danger does not exist with unreactive helium.
Noble gases are produced in different quantities depending on their frequency and availability. In 1998 the amount of argon produced was around 2 billion m 3 , while helium was isolated in an amount of around 130 million m 3 . The world annual production of xenon, on the other hand, is estimated at only 5,000–7,000 m 3 for 1998 ( standard cubic meters each ). The prices of the gases differ accordingly: Argon costs around 15 euros per cubic meter (under standard conditions, laboratory quality), xenon costs 10,000 euros per cubic meter (as of 1999).
links
Xenon compounds
The greatest variety of noble gas compounds is found with xenon. The most important and most stable are the xenon fluorides xenon (II) fluoride , xenon (IV) fluoride and xenon (VI) fluoride , which are synthesized by reacting xenon and fluorine in different ratios. Xenon (II) fluoride is the only noble gas compound that is used industrially in small quantities; it serves as a strong oxidizing and fluorinating agent in organic chemistry.
With oxygen, xenon reaches the highest possible oxidation level +8. This is achieved in xenon (VIII) oxide and the oxyfluoride xenon difluoride trioxide XeO 3 F 2 and in perxenates of the form XeO 4 - . Further, xenon (VI) oxide and the oxyfluorides XeO 2 F 2 and XeOF 4 in the oxidation state +6 and the oxyfluoride XeOF 2 tetravalent Xenon known. All xenon oxides and oxyfluorides are unstable and often explosive. Compounds of xenon with nitrogen, chlorine and carbon are also known. Complexes with metals such as gold or mercury could also be synthesized under super acidic conditions .
Compounds of other noble gases
Only a small number of compounds are known of the other noble gases. Radon compounds should be thermodynamically as stable as xenon compounds, but their synthesis and exact characterization are extremely difficult due to the strong radioactivity and short half-life of the radon isotopes. The existence of a stable radon (II) fluoride is assumed, since radon can no longer be detected after passing through liquid chlorine trifluoride and must therefore have reacted. If the residues of this solution are dissolved in water or acids, the decomposition products formed are oxygen and hydrogen fluoride in the same ratio as with krypton or xenon difluoride.
All known compounds of lighter noble gases are thermodynamically unstable, easily decompose and can therefore only be synthesized at low temperatures, if at all. The most important and most stable krypton compound is krypton (II) fluoride , which is one of the most powerful oxidizing and fluorinating agents known. Krypton (II) fluoride can be produced directly from the elements and is the starting product for a number of other krypton compounds.
While helium and neon compounds are still the only subject of theoretical investigations and calculations have shown that at most one helium compound (HHeF), but not a single neon compound, should be stable, a first argon compound has now actually been synthesized: by photolysis of hydrogen fluoride in one to 7.5 K cooled down argon matrix, the very unstable argon fluorohydride could be formed, which breaks down into its constituents again when two molecules come into contact or when heated above 27 K.
Clathrates
Argon, krypton and xenon form clathrates , inclusion compounds in which the noble gas is physically enclosed in a surrounding solid. Typical examples of this are noble gas hydrates, in which the gases are enclosed in ice . An argon hydrate forms slowly only at −183 ° C, hydrates of krypton and xenon already at −78 ° C. Noble gas clathrates are also known to exist with other substances such as hydroquinone .
literature
- P. Häussinger, R. Glatthaar, W. Rhode, H. Kick, C. Benkmann, J. Weber, H.-J. Wunschel, V. Stenke, E. Leicht, H. Stenger: Noble Gases. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2006 ( doi : 10.1002 / 14356007.a17_485 ).
- Entry on noble gases. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 417-429.
Web links
Individual evidence
- ↑ Horst Briehl: Chemistry of materials. 2nd edition 2007, Springer, ISBN 978-3-8351-0223-1 , p. 14.
- ^ A b c d William H. Brock: Viewegs history of chemistry. Vieweg, Braunschweig 1997, ISBN 3-540-67033-5 , pp. 211-216.
- ^ RK Kochhar: French astronomers in India during the 17th – 19th centuries. In: Journal of the British Astronomical Association. 1991, 101, pp. 95-100 ( article ).
- ↑ a b entry on helium. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ^ Lord Rayleigh: Density of Nitrogen . Letters to the Editor. In: Nature . tape 46 , no. 1196 , September 1892, p. 512-513 , doi : 10.1038 / 046512c0 (English, https://web.lemoyne.edu/~giunta/rayleigh0.html , https://archive.org/details/scientificpapers04rayliala/page/1 - The production method from ammonia was by Ramsay.): “density of nitrogen […] to two methods of preparation I obtain quite distinct values. The relative difference [...] can only be attributed to a variation in the character of the gas. "
- ^ Günther Bugge: The book of the great chemists , Verlag Chemie, Weinheim 1974, ISBN 3-527-25021-2 , p. 255.
- ↑ a b entry on radon. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ↑ a b Yu. Ts. Oganessian, VK Utyonkov, Yu. V. Lobanov, F. Sh. Abdullin, AN Polyakov, RN Sagaidak, IV Shirokovsky, Yu. S. Tsyganov, AA Voinov, GG Gulbekian, SL Bogomolov, BN Gikal, AN Mezentsev, S. Iliev, VG Subbotin, AM Sukhov, K. Subotic, VI Zagrebaev, GK Vostokin, MG Itkis : Synthesis of the isotopes of elements 118 and 116 in the 249 Cf and 245 Cm + 48 Ca fusion reactions . In: Phys. Rev. C. 2006, 74, 4, pp. 044602-1-044602-1, doi : 10.1103 / PhysRevC.74.044602 .
- ^ A b John F. Lehmann, Hélène PA Mercier, Gary J. Schrobilgen: The chemistry of krypton. In: Coordination Chemistry Reviews . 2002, 233/234, pp. 1-39, doi : 10.1016 / S0010-8545 (02) 00202-3 .
- ↑ Neil Bartlett: Xenon Hexafluoroplatinate (V) Xe + [PtF] - . In: Proceedings of the Chemical Society. 1962, p. 218, doi : 10.1039 / PS9620000197 .
- ^ R. Hoppe: The valence compounds of the noble gases. In: Angewandte Chemie. 1964, 76, 11, pp. 455-463, doi : 10.1002 / anie.19640761103 .
- ^ A b Leonid Khriachtchev, Mika Pettersson, Nino Runeberg, Jan Lundell, Markku Räsänen: A stable argon compound. In: Nature. 2000, 406, pp. 874-876, doi : 10.1038 / 35022551 .
- ^ AGW Cameron: Abundances of the elements in the solar system. In: Space Science Reviews , 1970, 15, pp. 121-146 ( PDF ).
- ^ A b David R. Williams: Earth Fact Sheet . NASA , Greenbelt, as of May 20, 2009.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Geophysics, Astronomy, and Acoustics, pp. 14-18.
- ↑ a b c d e f g h i j k l P. Häussinger, R. Glatthaar, W. Rhode, H. Kick, C. Benkmann, J. Weber, H.-J. Wunschel, V. Stenke, E. Leicht, H. Stenger: Noble Gases. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2006 ( doi : 10.1002 / 14356007.a17_485 ).
- ↑ Chris J. Ballentine: Geochemistry: Earth holds its breath. In: Nature . 2007, 449, pp. 294-296, doi : 10.1038 / 449294a .
- ↑ Klaus Hoffmann: Can you make gold? Crooks, jugglers and scholars. From the history of the chemical elements . Urania-Verlag, Leipzig Jena Berlin 1979, no ISBN, p. 67.
- ↑ a b c d e A. GM Ferreira, LQ Lobo: On the vapor pressure of radon. In: The Journal of Chemical Thermodynamics. 2007, 39, 10, pp. 1404-1406, doi : 10.1016 / j.jct.2007.03.017
- ↑ K. Schubert: A model for the crystal structures of the chemical elements . In: Acta Crystallographica . 1974, 30, pp. 193-204, doi : 10.1107 / S0567740874002469 .
- ^ AV Grosse: Some physical and chemical properties of element 118 (Eka-Em) and element 86 (Em). In: Journal of Inorganic and Nuclear Chemistry. 1965, 27, 3, pp. 509-519, doi : 10.1016 / 0022-1902 (65) 80255-X .
- ↑ Clinton S. Nash: Atomic and Molecular Properties of Elements 112, 114, and 118. In: J. Phys. Chem. A. 2005, 109, 15, pp. 3493-3500, doi : 10.1021 / jp050736o .
- ^ Wiese, WL: = Atomic, Molecular, and Optical Physics Handbook . Ed .: Drake, GWF American Institute of Physics, 1996, ISBN 1-56396-242-X (English).
- ↑ a b Errol G. Lewars: Modeling Marvels: Computational Anticipation of Novel Molecules. Springer Verlag, 2008, ISBN 978-1-4020-6972-7 , pp. 69-80.
- ^ LC Allen, JE Huheey: The definition of electronegativity and the chemistry of the noble gases. In: Journal of Inorganic and Nuclear Chemistry . 1980, 42, pp. 1523-1524, doi : 10.1016 / 0022-1902 (80) 80132-1 .
- ^ Hans-Hermann Braess, Ulrich Seiffert: Vieweg handbook automotive technology. 5th edition, Vieweg + Teubner Verlag, 2007, ISBN 978-3-8348-0222-4 , pp. 674-676.
- ^ Walter J. Moore, Dieter O. Hummel: Physikalische Chemie. 4th edition, de Gruyter, 1986, ISBN 978-3-11-010979-5 , p. 284.
- ^ Alfred A. Bove, Jefferson Carroll Davis: Bove and Davis' diving medicine. 4th edition, Elsevier, 2004, ISBN 978-0-7216-9424-5 , pp. 119-121.
- ^ T. Marx, M. Schmidt, U. Schirmer, H. Reinelt: Xenon anaesthesia. In: Journal of the Royal Society of Medicine. Volume 93, number 10, October 2000, pp. 513-517, doi : 10.1177 / 014107680009301005 , PMID 11064688 , PMC 1298124 (free full text) (review).
- ↑ Entry on xenon connections. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ↑ Lawrence Stein: Ionic Radon Solutions. In: Science. 1970, 168, pp. 362-364, doi : 10.1126 / science.168.3929.362 .
- ↑ Leonid Khriachtchev, Hanna Tanskanen, Arik Cohen, R. Benny Gerber, Jan Lundell, Mika Pettersson, Harri Kiljunen, Markku Räsänen: A Gate to Organokrypton Chemistry: HKrCCH. In: Journal of the American Chemical Society . 2003, 125, 23, pp. 6876-6877, doi : 10.1021 / ja0355269 .
- ^ RM Barrer, DJ Ruzicka: Non-stoichiometric clathrate compounds of water. Part 4. - Kinetics of formation of clathrate phases. In: Transactions of the Faraday Society. 1962, 58, pp. 2262-2271, doi : 10.1039 / TF9625802262 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-4.