Uranium (III) fluoride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ U 3+ __ F - | ||||||||||||||||
| Crystal system | ||||||||||||||||
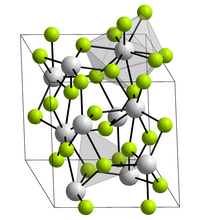
| Space group |
P 3 c 1 (No. 165) |
|||||||||||||||
| Lattice parameters |
a = 717.9 pm |
|||||||||||||||
| Coordination numbers |
U [9], F [3] |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Uranium (III) fluoride | |||||||||||||||
| other names |
Uranium trifluoride |
|||||||||||||||
| Molecular formula | UF 3 | |||||||||||||||
| Brief description |
purple solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 295.02 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
8.9 g cm −3 |
|||||||||||||||
| Melting point |
1495 ° C |
|||||||||||||||
| Hazard and safety information | ||||||||||||||||
 Radioactive |
||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
- (1508.5 ± 5.5) kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Uranium (III) fluoride is a chemical compound made up of the elements uranium and fluorine . It has the formula UF 3 and belongs to the fluoride class of substances .
presentation
Uranium (III) fluoride can be obtained by reacting uranium (IV) fluoride with aluminum at 900 ° C.
Production by reaction with a stoichiometric amount of uranium is also possible.
properties
Uranium (III) fluoride is a purple solid that melts at 1495 ° C. When heated to temperatures above 1200 ° C, it disproportionates to uranium (IV) fluoride and uranium. Its crystal structure is trigonal with the lattice parameters a = 717.9 pm and c = 734.5 pm. This corresponds to that of lanthanum fluoride . Each uranium ion is surrounded by nine fluoride ions in a distorted triple-capped trigonal-prismatic structure.
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1969.
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-97.
- ↑ Entry on uranium compounds in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry uranium compounds with the exception of those specified elsewhere in this Annex in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling.
- ↑ EHP Cordfunke, W. Ouweltjes: "Standard Enthalpies of Formation of Uranium Compounds VII. UF 3 and UF 4 (by Solution Calorimetry)", in: The Journal of Chemical Thermodynamics , 1981 , 13 (2), pp. 193-197 ( doi: 10.1016 / S0021-9614 (81) 80025-0 ).
- ↑ a b c Georg Brauer (ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 1207.
literature
- Ingmar Grenthe, Janusz Drożdżynński, Takeo Fujino, Edgar C. Buck, Thomas E. Albrecht-Schmitt, Stephen F. Wolf: Uranium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 253-698 ( doi : 10.1007 / 1-4020-3598-5_5 ).




