Sodium arsenate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
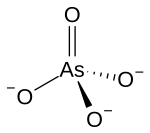
|
||||||||||
| General | ||||||||||
| Surname | Sodium arsenate | |||||||||
| other names |
Arsenic acid sodium salt |
|||||||||
| Molecular formula | Na 3 AsO 4 | |||||||||
| Brief description |
colorless and odorless solid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 207.89 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
|
|||||||||
| Melting point |
86 ° C |
|||||||||
| solubility | ||||||||||
| safety instructions | ||||||||||
|
||||||||||
| MAK |
Switzerland: 0.01 mg m −3 (based on arsenic, measured as inhalable dust ) |
|||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Sodium arsenate is a chemical compound of sodium from the group of arsenates . According to some sources, the compound with the composition AsH 3 O 4 · xNa is not precisely specified.
Extraction and presentation
Sodium arsenate can be obtained by reacting arsenic trioxide with sodium nitrate .
properties
Sodium arsenate is a crystalline, colorless and odorless solid that is soluble in water. It decomposes when heated above 180 ° C. Its structure (as dodecahydrate) is isomorphic to that of sodium phosphate . It has a hexagonal crystal structure with the space group P 3 c 1 (space group no. 165) .
use
Sodium arsenate is used as an insecticide in wood preservatives and ant baits .
Related links
- Sodium dihydrogen arsenate NaH 2 AsO 4
- Disodium hydrogen arsenate Na 2 HAsO 4
- Sodium metaarsenate NaAsO 3
- Sodium diarsenate Na 4 As 2 O 7
Individual evidence
- ↑ a b c d e f UK Poison Information Documents (UKPID) for Sodium arsenate , accessed October 16, 2016.
- ↑ a b c d e f g Entry on sodium arsenate, unspecified salt in the GESTIS substance database of the IFA , accessed on October 16, 2016(JavaScript required) .
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry arsenic compounds, with the exception of those named in this appendix in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) on February 6, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for arsenic and inorganic arsenic compounds ), accessed on March 4, 2020.
- ↑ Angel Vegas: Inorganic 3D Structures . Springer Science & Business Media, 2011, ISBN 978-3-642-20340-4 , p. 106 ( limited preview in Google Book search).
- ↑ R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 600 ( limited preview in Google Book Search).


