Sodium metaarsenate
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ Na + __ As 5+ __ O 2− | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium metaarsenate | |||||||||||||||
| other names |
Sodium polyarsenate |
|||||||||||||||
| Ratio formula | NaAsO 3 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 145.91 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
615 ° C |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium metaarsenate is a chemical compound from the group of arsenates .
Extraction and presentation
Sodium metaarsenate can be obtained by thermal decomposition of sodium dihydrogen arsenate above 230 ° C.
properties
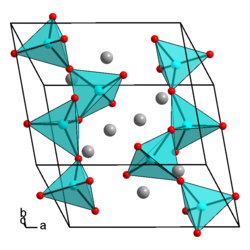
Sodium metaarsenate is a colorless solid that is in the form of needle-like crystals. The compound has a polymer structure. The compound has a triclinic crystal structure (a = 8.07, b = 7.44, c = 7.32 Å , α = 90 °, β = 91.5 °, γ = 104 °) with the space group P 1 (space groups No. 2) . Its structure corresponds to the Maddrell salt. Sodium metaarsenate has an X-ray density of 3.4 g · cm −3 and a pycnometric density of 3.54 g · cm −3 .
Individual evidence
- ↑ a b c d e f Liebau F .: About the crystal structure of sodium polyarsenate, (NaAsO3) x . In: Acta Crystallographica . tape 9 , no. 10 , October 10, 1956 ISSN 0365-110X , doi : 10.1107 / s0365110x56002199 .
- ↑ R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 600 ( limited preview in Google Book Search).
- ↑ a b c H.J.Emeléus & AG Sharpe: Advances in Inorganic Chemistry and Radiochemistry . Academic Press, 1962, ISBN 978-0-08-057853-8 , pp. 59 ( limited preview in Google Book search).
- ^ Entry on arsenic compounds in the GESTIS substance database of the IFA , accessed on February 22, 2019 (JavaScript required)
- ↑ Not explicitly listed in Regulation (EC) No. 1272/2008 (CLP) , but with the specified labeling it falls under the group entry arsenic compounds, with the exception of those named in this appendix in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) on February 6, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .


