Rubidium chlorate
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
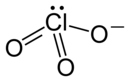
|
|||||||||||||
| General | |||||||||||||
| Surname | Rubidium chlorate | ||||||||||||
| Molecular formula | RbClO 3 | ||||||||||||
| Brief description |
white prisms |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 168.92 g · mol -1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
3.19 g cm −3 |
||||||||||||
| solubility |
21.38 g l −1 at 0 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Rubidium chlorate is the rubidium salt of chloric acid with the chemical composition RbClO 3 .
Manufacturing
Rubidium chlorate can be made from rubidium sulfate and barium chlorate .
properties
Physical Properties
The solubility of rubidium chlorate in water increases with increasing temperature and is shown in the table below.
| Solubility of RbClO 3 in Water | ||||||||||
| Temperature [° C] | 0 | 8th | 19.8 | 30th | 42.2 | 50 | 76 | 99 | ||
| Solubility [g / l] | 21.38 | 30.7 | 53.6 | 80 | 124.8 | 159.8 | 341.2 | 628 | ||
Rubidium chlorate crystallizes in the trigonal crystal system with the space group R 3 m (space group no. 160) and the lattice parameters a = 608.9 pm and c = 817.4 pm, there are three formula units in the unit cell .
Chemical properties
When heated, rubidium chlorate breaks down into rubidium chloride and oxygen :
Individual evidence
- ^ Wilhelm Steffen: Textbook of pure and technical chemistry: Inorganische Experimental-Chemie . J. Maier, 1893, p. 89. Full text
- ^ A b Dale L. Perry, Sidney L. Phillips: Handbook of inorganic compounds . CRC Press, 1995, ISBN 978-0-8493-8671-8 , p. 333 ( limited preview in Google book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ R. Abegg, F. Auerbach: "Handbuch der inorganic Chemie". Verlag S. Hirzel, Vol. 2, 1908. P. 431. Full text
- ↑ Aterton Seidell: "Solubilities Of Organic Compounds Vol - I", S. 1429. Full text
- ^ Jean D'Ans, Ellen Lax: Pocket book for chemists and physicists. 3. Elements, inorganic compounds and materials, minerals, Volume 3. 4. Edition, Springer, 1997, ISBN 978-3-5406-0035-0 , p. 686 ( limited preview in the Google book search).

