Calcium iodate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
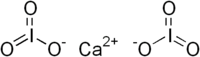
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Calcium iodate | |||||||||||||||
| Molecular formula | Ca (IO 3 ) 2 | |||||||||||||||
| Brief description |
White dust |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 388.89 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
4.519 g cm −3 |
|||||||||||||||
| solubility |
heavy in water (2.4 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Calcium iodate is a chemical compound consisting of the elements calcium , iodine and oxygen with the empirical formula CaI 2 O 6 . It is the calcium salt of iodic acid .
Calcium iodate occurs naturally as the mineral Lautarite . It crystallizes monoclinically in the crystal class 2 / m.
Extraction
Calcium iodate can be obtained from calcium iodide by oxidation .
use
Calcium cadmium iodate is used as an antiseptic in medicine and deodorants .
Individual evidence
- ↑ a b Entry on Calcium iodate in the Hazardous Substances Data Bank , accessed April 13, 2013.
- ↑ a b c Mineralienatlas: Lautarit (Wiki)
- ↑ a b Entry on calcium iodate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Growth and study of mixed crystals of Ca – Cd iodate (PDF; 296 kB)

