Sodium permanganate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
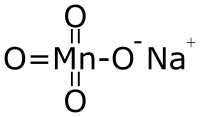
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium permanganate | |||||||||||||||
| Molecular formula | NaMnO 4 | |||||||||||||||
| Brief description |
purple odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 141.93 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.47 g cm −3 |
|||||||||||||||
| solubility |
very light in water (1440 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium permanganate is a chemical compound from the group of sodium compounds and permanganates .
Extraction and presentation
Sodium permanganate can be obtained by reacting manganese dioxide with sodium hypochlorite .
properties
Sodium permanganate is a purple, odorless, oxidizing solid that is soluble in water. It decomposes when heated, producing oxygen .
use
Due to its high solubility, its aqueous solutions are used as an etchant for printed circuit boards.
In the V2 rocket it was used for propulsion through the catalytic decomposition of highly concentrated hydrogen peroxide .
Sodium permanganate is also used in the industrial production of cocaine . That is why the US Drug Enforcement Agency (DEA) has restricted sales since 2006, which led to the price of cocaine doubling.
Individual evidence
- ↑ a b c d e f Entry on sodium permanganate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Arno H. Reidies: Manganese compounds . In: Ullmann's Encyclopedia of Industrial Chemistry , 2002, Wiley-VCH, Weinheim ( doi : 10.1002 / 14356007.a16_123 ).
- ↑ Doug Carroll: Why cocaine prices went up 100% in the US , March 15, 2015, accessed January 28, 2018.






