Sodium manganate
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
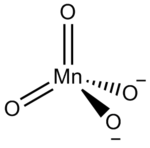
|
|||||||
| General | |||||||
| Surname | Sodium manganate | ||||||
| other names |
Sodium manganate (VI) |
||||||
| Molecular formula | Na 2 MnO 4 | ||||||
| Brief description |
dark green solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 164.914 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Sodium manganate , Na 2 MnO 4 , is a chemical compound of sodium from the group of manganates .
Extraction and presentation
To produce sodium manganate , the manganese (II) is oxidized by the sodium peroxide to form the corresponding manganate (VI).
Individual evidence
- ^ Douglas M. Considine, Glenn D. Considine: Van Nostrand's Scientific Encyclopedia . Springer Science & Business Media, 2013, ISBN 978-1-4757-6918-0 , p. 2879 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Comprehensive Chemistry XI . Laxmi Publications, ISBN 81-7008-596-9 , pp. 868 .
