Rubidium amide
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
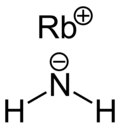
|
|||||||
| General | |||||||
| Surname | Rubidium amide | ||||||
| Molecular formula | RbNH 2 | ||||||
| Brief description |
white deliquescent crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 101.49 g · mol -1 | ||||||
| Physical state |
firmly |
||||||
| density |
2.59 g cm −3 |
||||||
| Melting point |
309 ° C |
||||||
| solubility |
reacts with water |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Rubidium amide is a chemical compound made of rubidium , nitrogen and hydrogen .
Manufacturing
Rubidium amide is formed when the metal is heated in the ammonia stream.
It is also formed by the reaction of rubidium hydride with liquid ammonia with evolution of hydrogen. The reaction with gaseous ammonia takes place very slowly at room temperature.
When heating rubidium in a nitrogen stream arises also Rubidiumamid, as a byproduct, however, is in no small quantities Rubidiumnitrid .
properties
Physical Properties
Rubidium amide crystallizes in the cubic crystal system in the space group Fm 3 m (space group no. 225) with the lattice parameter a = 639.5 pm.
Chemical properties
Rubidium amide reacts with water to form ammonia and rubidium hydroxide .
With ethanol to form Rubidiumethanolat and ammonia.
Individual evidence
- ↑ a b c R. Abegg, F. Auerbach: 'Handbuch der inorganic Chemie'. Verlag S. Hirzel, Vol. 2, 1908. P. 430. Full text
- ↑ a b c d e Jean D'Ans, Ellen Lax: Paperback for chemists and physicists. 3. Elements, inorganic compounds and materials, minerals, Volume 3. 4. Edition, Springer, 1997, ISBN 978-3-5406-0035-0 , pp. 688f. ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ H. Moissan: "Preparation et propriétés des hydrures de rubidium et de césium" in Compt. Rend. Hebd. 1903 , 136 , p. 587. Full text



