Cerium (IV) sulfate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
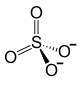
|
||||||||||
| General | ||||||||||
| Surname | Cerium (IV) sulfate | |||||||||
| other names |
Cerium disulfate |
|||||||||
| Molecular formula | Ce (SO 4 ) 2 | |||||||||
| Brief description |
orange solid (tetrahydrate) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 332.23 g mol −1 (anhydrous) | |||||||||
| Physical state |
firmly |
|||||||||
| density |
5.02 g cm −3 (20 ° C) |
|||||||||
| Melting point |
180–200 ° C (release of crystal water) |
|||||||||
| solubility |
moderate in water (38 g l −1 at 50 ° C) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Cerium (IV) sulfate or cerium disulfate is a salt of the rare earth metal cerium with sulfuric acid .
presentation
It is represented by the conversion of cerium dioxide with conc. Sulfuric acid with heating:
properties
Like all cerium (IV) salts, cerium (IV) sulfate is a strong oxidizing agent, especially under acidic conditions. Under these conditions, cerium (IV) sulfate releases elemental chlorine from dilute hydrochloric acid ; even if the response speed is slow. However, it reacts much faster with strong reducing agents.
Cerium compounds occur in the +3 and +4 oxidation states. Following this monovalence, cerium (IV) sulfate reacts when reacting with reducing agents to form Ce (III):
use
Because of its oxidative property in combination with the monovalence and the resulting uniqueness of the reaction, cerium (IV) sulfate is used in redox titrimetry ( cerimetry ), e.g. B. to determine the content of paracetamol according to the European Pharmacopoeia. Customized solutions are available commercially for such applications.
It is also used in organic synthesis, for example the oxidation of 1,2- glycols or the quinone synthesis.
literature
- Arnold Willmes: Text book chemical substances . 1st edition. Self-published by Dr. Willmes GmbH, Thun / Frankfurt am Main 1990.
Individual evidence
- ↑ a b c d data sheet cerium (IV) sulfate (PDF) from Merck , accessed on January 20, 2013.
- ↑ a b Entry on cerium (IV) sulfate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)



