Potassium selenate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
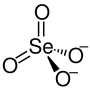
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Potassium selenate | |||||||||||||||
| other names |
Potassium orthoselenate |
|||||||||||||||
| Molecular formula | K 2 SeO 4 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 221.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.07 g cm −3 |
|||||||||||||||
| Melting point |
1002 ° C |
|||||||||||||||
| solubility |
114 g / l at 25 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−1110 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Potassium selenate is an inorganic chemical compound of potassium from the selenate group .
Extraction and presentation
Potassium selenate can be obtained by allowing selenium dioxide to react with a cooled potassium hydroxide solution and oxidizing the potassium selenite obtained in this way with bromine in an alkaline medium.
properties
Potassium selenate is a white, odorless solid that is soluble in water. Four modifications are known of the compound and, at room temperature, has an orthorhombic crystal structure with the space group Pnma (space group no. 62) . At low temperatures below 93 K it is in a different, ferroelectric modification (space group Pna 2 1 (No. 33) ). At a temperature above 745 K it changes into a hexagonal crystal structure with the space group P 6 3 / mmc (space group no. 194) .
use
Potassium selenate can be used to make selenium trioxide . Potassium selenate has also been used to treat selenium deficiency in farm animals.
Individual evidence
- ↑ a b c d e f data sheet Potassium selenate from AlfaAesar, accessed on June 15, 2016 ( PDF )(JavaScript required) .
- ^ A b Evgenii Tonkov: High Pressure Phase Transformations A Handbook . CRC Press, 1992, ISBN 978-2-88124-759-0 , pp. 518 ( limited preview in Google Book search).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Inorganic Compounds, pp. 4-83.
- ↑ M. Binnewies, E. Mike: Thermochemical Data of Elements and Compunds . 2nd Edition. Wiley-VCH, Weinheim 2002, ISBN 3-527-30524-6 , chap. 5 , p. 645 .
- ↑ Irene Rosenfeld, Orville A. Beath: Selenium Geobotany, Biochemistry, Toxicity, and Nutrition . Academic Press, 2013, ISBN 978-1-4832-7590-1 , pp. 305 ( limited preview in Google Book Search).
- ↑ N. Yamada, Y. Ono, T. Ikeda: A Structural Study of the Incommensurate-to-Ferroelectric Phase Transition in K 2 SeO 4 . In: Journal of the Physical Society of Japan , 53, 1984, pp. 2565-2574, doi: 10.1143 / JPSJ.53.2565 .
- ^ N. Ohama: Superstructure of potassium selenate K2SeO4. In: Materials Research Bulletin. 9, 1974, p. 283, doi : 10.1016 / 0025-5408 (74) 90078-6 .
- ^ A. Kálmán, JS Stephens, DWJ Cruickshank: The crystal structure of K2SeO4. In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 26, p. 1451, doi : 10.1107 / S0567740870004314 .
- ↑ Hermann Sicius: chalcogens: Elements of the sixth main group A journey through the periodic table . Springer-Verlag, 2015, ISBN 978-3-658-10522-8 , pp. 28 ( limited preview in Google Book search).
- ↑ and farm animals: Justified by W. Löscher, FR ...: Pharmacotherapy in domestic and farm animals Justified by W. Löscher, FR Ungemach and R. Kroker . Georg Thieme Verlag, 2014, ISBN 3-8304-1252-5 ( limited preview in Google book search).
- ↑ Entry on potassium selenate at Vetpharm, accessed on June 15, 2016.




