Dysprosium (III) chloride
| Crystal structure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| __ Dy 3+ __ Cl - | ||||||||||
| Space group |
C 2 / m (No. 12) |
|||||||||
| General | ||||||||||
| Surname | Dysprosium (III) chloride | |||||||||
| other names |
Dysprosium trichloride |
|||||||||
| Ratio formula | DyCl 3 | |||||||||
| Brief description |
|
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 268.86 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| density |
3.67 g cm −3 (25 ° C) |
|||||||||
| Melting point |
|
|||||||||
| boiling point |
1530 ° C |
|||||||||
| solubility |
soluble in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Dysprosium (III) chloride is a chemical compound from the group of chlorides .
Extraction and presentation
Dysprosium (III) chloride can be synthesized directly from the elements dysprosium and chlorine .
The hexahydrate can be obtained by reacting dysprosium (III) oxide , dysprosium or dysprosium (III) carbonate with hydrochloric acid .
properties
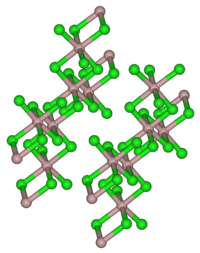
Dysprosium (III) chloride is present as yellowish-white, mother-of-pearl-like, shiny scales. It has a monoclinic crystal structure with the space group C 2 / m (space group no.12) corresponding to that of aluminum (III) chloride with the lattice parameters a = 691 pm, b = 1197 pm, c = 640 pm, β = 111, 28 ° and Z = 2. The hexahydrate is a light yellow solid.
use
Dysprosium (III) chloride can be used as a catalyst in organic syntheses.
literature
- Tomasz Mioduskia, Cezary Gumińskib, Dewen Zengc: IUPAC-NIST Solubility Data Series. 87. Rare Earth Metal Chlorides in Water and Aqueous Systems. Part 3. Heavy Lanthanides (Gd – Lu) . In: Journal of Physical and Chemical Reference Data . tape 38 , no. 4 , 2009, p. 925-1011 , doi : 10.1063 / 1.3212962 .
Individual evidence
- ↑ a b c d e Jean D'Ans, Ellen Lax: Paperback for chemists and physicists . 2007, ISBN 978-3-540-60035-0 , pp. 442 ( limited preview in Google Book search).
- ↑ a b c data sheet Dysprosium (III) chloride hexahydrate, 99.9% trace metals basis from Sigma-Aldrich , accessed on April 29, 2012 ( PDF ).
- ↑ a b c d e f data sheet Dysprosium (III) chloride, anhydrous, powder, 99.99% trace metals basis from Sigma-Aldrich , accessed on April 29, 2012 ( PDF ).
- ↑ Web elements: Dysprosium
- ↑ S. Cahen, R. Vangelisti: Synthesis, structure and magnetic properties of lanthanide trichlorides-GIC: Stage-2 DyCl3-GIC. In: Journal of Physics and Chemistry of Solids . 67, No. 5-6, 2006, pp. 1223-1227, doi: 10.1016 / j.jpcs.2006.01.094 .
- ↑ Gesine K. Veits, Javier Read de Alaniz: Dysprosium (III) catalysis in organic synthesis. In: Tetrahedron . 68, No. 8, 2012, pp. 2015–2026, doi: 10.1016 / j.tet.2011.11.042 .





