Aluminum hydride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
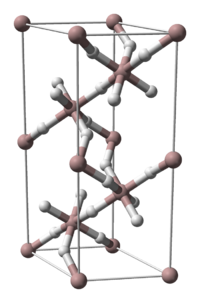
|
|||||||||||||||||||
| __ Al 3+ __ H - | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aluminum hydride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | (AlH 3 ) x | ||||||||||||||||||
| Brief description |
colorless powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 30.01 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.48 g cm −3 |
||||||||||||||||||
| Melting point |
100 ° C (decomposition) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−46.0 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aluminum hydride (Alan) is a chemical compound made up of the elements aluminum and hydrogen with the ratio formula (AlH 3 ) x . It is a colorless powder that breaks down into its components above 100 ° C. Aluminum hydride is extremely sensitive to moisture and oxidation. It burns explosively in air and can be used to store hydrogen in hydrogen-powered vehicles.
properties
Five crystalline phases (α, γ, δ, ζ) are known of aluminum hydride, of which only the structure of the α phase has so far been researched. The vacuum stability of α-aluminum hydride can be significantly increased by incorporating 0.01 to 3 percent by weight of magnesium . The resistance to hydrolysis can also be increased by tempering . Α-aluminum hydride treated in this way has been intensively investigated as an energetic additive for rocket fuels and explosives.
synthesis
Aluminum hydride is expediently prepared by reacting aluminum chloride with lithium aluminum hydride in diethyl ether :
Here, a compound that occurs as a monomer , the etherate:
which is gradually converted into the high polymer aluminum hydride.
Responsiveness
Aluminum hydride reacts very strongly with water releasing hydrogen according to:
Together with other metal hydrides, aluminum hydride forms alanates . The most important representative is lithium aluminum hydride (lithium alanate):
Because of its insolubility , it is of little importance as a reducing agent.
The dimer Al 2 H 6 is isostructural to diborane (B 2 H 6 ) and digallane (Ga 2 H 6 ).
swell
- ↑ a b Entry on aluminum hydride. In: Römpp Online . Georg Thieme Verlag, accessed on July 14, 2014.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of aluminum trihydride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on July 16, 2018, is reproduced from a self-classification by the distributor .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-5.
- ^ N. E: Matzek, HC Roehrs, US Patent 3,857,922 , 1975 , Stabilization of light metal hydride .
- ↑ TO Golubkov, RU Patent 2175637, Method of Increasing thermal stability of aluminum hydride .
- ^ V. Weiser, N. Eisenreich, A. Koleczko, E. Roth: On the Oxidation and Combustion of AlH3 a Potential Fuel for Rocket Propellants and Gas Generators . Propellants, Explosives, Pyrotechnics, 2007, 32 (3), 213-212; doi : 10.1002 / prep.200700022 .
- ^ Guttmann / Hengge, Inorganic Chemistry , VCH, Weinheim, New York, Basel, Cambridge 1990.





