Diborane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Diborane | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | B 2 H 6 | ||||||||||||||||||
| Brief description |
colorless gas with a repulsively sweet odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 27.67 g mol −1 | ||||||||||||||||||
| Physical state |
gaseous |
||||||||||||||||||
| density |
1.17 kg m −3 (15 ° C, 1 bar) |
||||||||||||||||||
| Melting point |
−164.85 ° C |
||||||||||||||||||
| boiling point |
−92.5 ° C |
||||||||||||||||||
| Vapor pressure |
2.8 M Pa (0 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
Switzerland: 0.1 ml m −3 or 0.1 mg m −3 |
||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
36.4 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Diborane is a chemical compound made up of the elements boron and hydrogen . It has the empirical formula B 2 H 6 and is the simplest compound from the class of boron-hydrogen compounds ( boranes ).
history
Diborane was first synthesized in the 19th century by hydrolysis of metal borides, but has not been studied in depth. From 1912 to 1936, the great pioneer of borohydride chemistry, Alfred Stock, researched these compounds, leading to methods for the synthesis and handling of the very reactive, volatile and often toxic boron hydrides. He first proposed a structure for diborane similar to ethane . The diffraction of electron beams according to experiments by SH Bauer supported the structure proposed by him.
In the communication between HI Schlesinger and L. Pauling (who also adopted the ethane structure), the three-center bond was not explicitly discussed in his classic review in the early 1940s. However, the review discussed the C 2v structure in depth: "It can be seen that this assumption easily explains many of the chemical properties of diboranes ...".
In 1943, the undergraduate student at Balliol College , Oxford, Christopher Longuet-Higgins, together with RP Bell, published the now accepted structure of diborane. However, this was already described in 1921 in what was then the Soviet Union. In the years following the Longuet-Higgins / Bell proposal, there was extensive discussion of the correct structure. The debate ended in 1951 with the electron diffraction measurements by K. Hedberg and V. Schomaker, which confirmed the structure of diborane, which is still recognized today.
William Nunn Lipscomb Jr. confirmed the molecular structure of boranes by X-ray structure analysis in the 1950s and developed theories to explain this bond. He later used the same methods for similar problems, including the structure of carboranes . Lipscomb received the Nobel Prize in Chemistry in 1976 for his efforts .
Occurrence
Diborane does not occur naturally.
Extraction and presentation
There are several ways to produce diborane:
Reaction of lithium hydride with boron trifluoride :
Reaction of lithium tetrahydroaluminate with boron trichloride in diethyl ether :
Reaction of sodium boronate with boron trifluoride in ethylene glycol dimethyl ether :
Reaction of sodium boronate with iodine in ethylene glycol dimethyl ether or THF :
Reaction of potassium borohydride with phosphoric acid:
Technical manufacturing
Borane is technically hydrogenation of diboron trioxide (B 2 O 3 ) by means of metallic aluminum and aluminum chloride as the reducing agent at temperatures above 150 ° C and at a hydrogen pressure produced bar of 750th
properties
Diborane is a colorless, flammable, highly toxic gas with a pungent, sweet, disgusting odor. It is a compound that is metastable under standard conditions . Above 50 ° C begins its decomposition to hydrogen and higher boranes ( tetraborane , pentaborane , hexaborane , decaborane , and others). The ignition temperature of pure diborane is 145 ° C. If it contains traces of higher boranes, it can spontaneously self-ignite in the air and burns with strong heat generation.
In practice, it ignites at around 45 ° C. With an air volume fraction of 0.8 to 88% it forms explosive mixtures. Since the gas must therefore be handled with care, it is usually allowed to react with amines ( ) to form aminoborane complexes .
These (liquid) substances serve as a precursor for diborane and can be stored and transported without danger. It is recovered by adding strong acids (e.g. hydrochloric acid ).
When dissolved in water, diborane reacts with it. On a laboratory scale, diborane can be prepared in diglyme by oxidation of sodium borohydride with iodine .
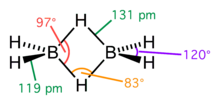
structure
Diborane is the simplest compound from the borane class, since monomeric borane (BH 3 ) is not stable and forms the metastable adduct diborane. The boron cores are tetrahedrally surrounded by four hydrogen nuclei. The two bridging hydrogen nuclei form a 2-electron-3-center bond in order to compensate for the electron deficiency in the boron nuclei. Two electrons are in an orbital distributed over three atoms .
Chemical properties
The boron in the diborane has an electron deficiency due to the two-electron three-center bonds to the two bridging hydrogen atoms. As a result, diborane is a monobasic Lewis acid and reacts with the formation of coordinative bonds to form Lewis bases (such as ammonia) and splits into monomeric boranes. With gaseous ammonia, BH 3 · NH 3 is formed accordingly .
The salts of diborane contain the monoboranate / tetrahydroboranate / tetrahydridoboranate ion BH 4 - and are called monoboranates / tetrahydroboranates. An important representative is by reacting diborane with sodium hydride resulting sodium borohydride .
use
Diborane is the most important reagent for hydroboration , whereby alkenes (R is any radical ) are linked via the B – H bonds to form trialkylboranes.
This reaction is regioselective and the resulting trialkylboranes can easily be converted into other useful organic derivatives.
Diborane is also used as a reducing agent, for example as a supplement to the reactivity of lithium aluminum hydride . The compound easily reduces carboxylic acids to the corresponding alcohols . It has also been considered as a rocket fuel , but it turned out to be completely unsuitable because it forms boron trioxide when burned , which clogs the engines. Diborane is still used for rubber vulcanization . Boron compounds are widely used as catalysts in the polymerization of hydrocarbons or in the manufacture of anti-Markovnikov products .
safety instructions
Diborane inhalation causes coughing, sore throat, dizziness, difficulty breathing, nausea and fatigue. It is highly toxic and extremely flammable.
Individual evidence
- ↑ a b c d e f g Entry on diborane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 19287-45-7 or diborane ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 97th edition. (Internet version: 2016), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-13.
- ↑ A. floor: The hydride of Boron and Silicon . Cornell University Press, New York 1933.
- ^ SH Bauer: The Structure of Diborane . In: Journal of the American Chemical Society . tape 59 , 1937, pp. 1096 , doi : 10.1021 / ja01285a041 .
- ^ SH Bauer: Structures and Physical Properties of the Hydrides of Boron and of their Derivatives . In: Chemical Reviews . tape 31 , 1942, pp. 43-75 , doi : 10.1021 / cr60098a001 .
- ^ HI Schlesinger, AB Burg: Recent Developments in the Chemistry of the Boron Hydrides . In: Chemical Reviews . tape 31 , 1942, pp. 1-41 , doi : 10.1039 / JR9430000250 .
- ^ HC Longuet-Higgins, RP Bell: The structure of the boron hydrides . In: Journal of the Chemical Society . 1943, p. 250-255 , doi : 10.1039 / JR9430000250 .
- ↑ W. Dilthey: rotary burner with a fixed gas supply . In: Journal of Applied Chemistry . tape 34 , 1921, pp. 594 , doi : 10.1002 / anie.19210349504 .
- ^ BV Nekrassov: J Gen Chem USSR . tape 10 , 1940, p. 1021 .
- ^ BV Nekrassov: J Gen Chem USSR . tape 10 , 1940, p. 1056 .
- ↑ K. Hedberg, V. Schomaker: A Reinvestigation of the Structures of Diborane and Ethane by Electron Diffraction . In: Journal of the American Chemical Society . tape 73 , 1951, pp. 1482–1487 , doi : 10.1021 / ja01148a022 .
- ↑ FA Cotton, G. Wilkinson: Inorganic Chemistry: A Summary for Advanced Students. trans. v. Heinz P. Fritz. 4., completely reworked. Edition. first reprint. VCH, Weinheim 1985.
- ^ ASB Prasad, JVB Kanth, M. Periasamy: Tetrahedron. 1992, 48, pp. 4623-4628.
- ^ Arlan D. Norman and William L. Jolly: Diborane . In: William L. Jolly (Ed.): Inorganic Syntheses . tape 11 . McGraw-Hill Book Company, Inc., 1968, p. 15-19 (English).
- ↑ James Arthur Campbell: General Chemistry: Energetics, Dynamics and Structure of Chemical Systems. 2nd, revised edition. Verlag Chemie (VCH), Weinheim 1985.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1066.



![\ mathrm {6 \ LiH \ + \ 8 \ BF_3 \ rightleftharpoons 6 \ Li [BF_4] \ + \ B_2H_6}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8c91c9fcb9a12fe5af77b0b149f10bd89c4027da)
![\ mathrm {3 \ Li [AlH_4] \ + \ 4 \ BCl_3 \ rightleftharpoons 3 \ Li [AlCl_4] \ + \ 2 \ B_2H_6}](https://wikimedia.org/api/rest_v1/media/math/render/svg/98f9563bf7a712573f1cbb04f54198062c828d72)