Tetraborane
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
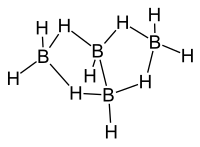
|
|||||||
| General | |||||||
| Surname | Tetraborane | ||||||
| other names |
|
||||||
| Molecular formula | B 4 H 10 | ||||||
| Brief description |
unpleasant smelling gas |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 53.32 g mol −1 | ||||||
| Physical state |
gaseous |
||||||
| Melting point |
−120 ° C |
||||||
| boiling point |
17.6 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Tetraborane , more precisely tetraborane-10, is an inorganic chemical compound from the group of boranes .
Extraction and presentation
Tetraborane produced by decomposition of diborane at temperatures above 50 ° C, whereby hydrogen and higher boranes ( pentaborane , hexaborane , decaborane , and others) are formed. A yield of up to 95% is achieved at a pressure of 170 kPa between two concentric glass tubes, of which the inner one is heated to 120 ° C and the outer one is cooled to −78 ° C (“hot-cold reactor”). It is also formed during the hydrolysis of magnesium diboride .
It can also be obtained by reacting iododiborane with sodium .
properties
Tetraborane is an unpleasant smelling gas. Pure tetraborane does not ignite in air, but is hydrolyzed to boric acid and hydrogen by water . It decomposes to other boranes in a few hours at room temperature (more rapidly at higher temperatures). Up to 100 ° C it breaks down mainly into hydrogen, diborane and pentaborane-9, at higher temperatures into hydrogen, pentaborane-9, hexaborane and boron-rich hydrides. It is attacked by bromine and chlorine much more slowly than diborane.
Individual evidence
- ↑ a b c Entry on tetraborane (10). In: Römpp Online . Georg Thieme Verlag, accessed on September 5, 2017.
- ↑ a b c d e Karl A. Hofmann: Inorganische Chemie . Springer-Verlag, 2013, ISBN 978-3-663-14240-9 , pp. 400 ( limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Karl A. Hofmann: Textbook of the inorganic experimental chemistry . Springer-Verlag, 2013, ISBN 978-3-663-04369-0 , pp. 383 ( limited preview in Google Book search).

