Cosmochemistry

The Cosmochemistry - even Astrochemistry called - is concerned with the formation and distribution of chemical elements and compounds in the universe . The chemical elements arise in the interior of stars ( nucleosynthesis ), chemical compounds, however, in cosmic gas and dust clouds , on planemos , moons , comets , asteroids and similar, colder objects.
Cosmochemistry is a modern, important branch of physics and chemistry and is strongly linked to astrophysics , especially the physics of stars and supernovae . It also plays a major role in planetology and in the attempt to understand the origin and chemical development of our solar system and other planemos (up to the origin of life - see under chemical evolution ).
However, since stars and almost all other celestial bodies are in an unreachable distance for us, one is restricted to certain methods with regard to chemical analysis , primarily instrumental methods of spectroscopy and (spectral) analysis , in which the radiation coming from the objects ( ultraviolet , visible light , infrared ) is evaluated.
Specifically, the Cosmochemistry not involved in the nucleosynthesis, but with the elemental and isotopic distribution in our solar system: A large contribution to this comes from the meteorite research , since meteorites have still the original chemical composition of the beginnings of the formation of our solar system. But also from (unmanned) space travel, a few samples of extraterrestrial material from the moon, comet dust, solar wind and - one hopes - samples from other planets, moons and asteroids will also be accessible in a few years and decades.
Cosmochemistry was founded in the 1950s by Friedrich-Adolf Paneth .
Example: A “cosmochemical” project
The way in which cosmochemistry works (astrophysics, astrochemistry, planetology) can be illustrated using a more recent example from unmanned space travel. Usually cosmochemists work on the evaluation of a spectral analysis or spectroscopy : Here they can use radiation spectra of the "light" of distant celestial bodies (mostly stars) to infer their chemical composition.
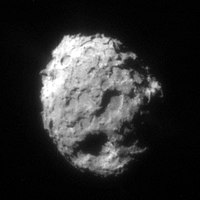
Since the first moon landings, samples of extraterrestrial material can now also be captured by space probes directly from space and brought to earth for direct analysis. The NASA mission " Stardust " made it possible not only to photograph the asteroid Annefrank and the comet Wild 2 , but also to capture cometary dust. On January 2, 2004, Stardust flew past the comet Wild 2 at a distance of 240 km and at a relative speed of 6.1 km / s. The probe took several images of the comet and collected its coma material. After returning to Earth, the Stardust capsule was brought to the NASA control center in Houston and opened there. In Houston, the condition of the airgel was tested , an extremely light solid in which the dust particles from comet Wild 2 were slowed down and transported. A small amount of comet dust was then made available to various groups of scientists - for example the Institute for Planetology in Münster . There the chemical composition of the dust particles could be examined directly. It is hoped that this will provide new insights into the formation of our solar system 4.6 billion years ago, because comet Wild 2 had only moved in the outer areas since the beginnings of the solar system (it was not until 1974 that the gravity of the giant planet Jupiter made it out of its old one Thrown track). On comets, researchers of cosmochemistry had found complex carbon compounds using spectral analysis during previous missions . They are not yet to be equated with life, but perhaps they gave the impetus for the origin of life on earth.
The formation of the chemical elements

The formation of the atomic nuclei ( nuclides ) and the chemical elements are described in detail in the article Nucleosynthesis . As a result, the chemical elements hydrogen and helium were created directly after the Big Bang through primordial nucleosynthesis. All heavier types of atoms then formed inside the fixed stars (stellar nucleosynthesis) and during supernova explosions. Towards the end of their burning time, very massive stars explode and hurl large quantities of heavy elements into space. For example, the supernova SN 2006gy in the galaxy NGC 1260 had 150 solar masses and when it exploded it blew an estimated 20 solar masses of nickel into the universe.
The distribution of the elements in the cosmos
The cosmochemical frequency distribution of the types of atoms can be explained in accordance with the history of the origins of the elements described under “ nucleosynthesis ”. The element abundance differs depending on the area you are looking at: however, by far the most common element in the entire universe is hydrogen - it is rather rare on earth, but found again frequently in humans.
Hydrogen and helium dominate in space , as both were created during the Big Bang . Of 1000 atoms in the universe, 900 are hydrogen atoms, and another 99 atoms are helium atoms. So just one atom in 1000 is not hydrogen or helium. All other types of atoms (except for lithium , beryllium and boron ) were formed in stars (see above and under nucleosynthesis). At the same time, atoms with an even number of protons were formed, for example oxygen , neon , iron or sulfur , which are therefore more common compared to other elements with an odd number of protons.
For every 1 billion hydrogen atoms (H) - i.e. 10 12 H atoms each - there are 10 10.8 helium atoms, 10 8.8 oxygen atoms, 10 8.6 carbon atoms and 10 8.0 nitrogen atoms, but in addition to about 10 7, 9 iron and neon atoms and 10 7.4 silicon atoms also only 10 1.7 lead, 10 0.7 gold and 10 0.3 silver atoms. The metallicity of the stars of the 1st generation (Population II) with an old age of over 10 billion years is different: They have a total of 1/1000 heavier elements than the "normal distribution" in space given here.
Interstellar matter - cosmochemically speaking
Interstellar matter contains the chemical elements in similar distributions as our sun and other stars of population I. Here, however, the atoms have their outer electrons due to lower temperatures, so that chemical compounds, gases and dusts, can arise between the star systems.
Between the stars there is hydrogen gas (neutral) with a density of 0.8 H atoms / cm 3 or 1.3 × 10 −24 g / cm 3 . Some areas are poorer in hydrogen (galactic center), in other places there are densities (fog, clouds) - and occasionally there even luminous areas, stimulated to glow by e.g. T. intense UV radiation of neighboring stars (emission nebula) or reflection (reflection nebula).
In the state of equilibrium between the speed of production and the rate of decay, complex, organic molecules are now formed in some nebulae, which, however, are often immediately broken down again by ionizing cosmic radiation. Nevertheless, they do exist and, shielded by clouds of dust, molecules such as water, ammonia, methane and formaldehyde (methanal) can have lifetimes of decades, nitrogen and carbon monoxide even 1000 years. They can also survive for long periods of time (up to 100,000 years) by freezing out on the surface of the dust grains. Even at densities of only 50 atoms / cm 3 , atomic collisions can produce molecules such as hydrogen and carbon monoxide, hydroxyl radicals or monocyan (CN).

In meteorites , cosmochemists even found alkanes such as 2,6,10,14-tetramethylpentadecane, aromatics such as benzene, toluene, xylenes and naphthalene, fatty acids with 14–28 carbon atoms, thiophenes, p -dichlorobenzene, amino acids such as proline, aspartic acid, Glycine, alanine and glutamic acid (Meteorit Murchison, 1969) and even adenine and guanine. The discovery of amino acids of extraterrestrial origin in 1970 was regarded as a definite sensation, as they are the basic building blocks of earthly life.
The formation of these organic molecules is explained by several mechanisms. Miller and Urey irradiated gas mixtures of methane, ammonia and water. Ions and radicals produced by radiolysis form ions with up to seven carbon atoms. Polymers can then even grow via ethene, and even carboxyl and amino groups can be incorporated via radicals such as NH 2 * and H 2 O * and react to form amino acids according to several mechanisms:
- the cyanohydrin mechanism (alkanal + ammonia + hydrocyanic acid to: nitrile + water, further reaction of the nitrile R-CH (CN) NH 2 with water to form the amino acid),
- according to Sanchez (NC-CCH + ammonia to NC-CH = CH-NH 2 + HCN and further with water with elimination of ammonia to asparagine ),
- via the Fischer-Tropsch synthesis (CO reacts with hydrogen at 10 −6 to 10 −2 atm and 450–750 Kelvin to methane or higher alkanes and water, catalyzed by Ni, Fe, magnetite and / or water-containing silicates on the dust grains - and with a cosmic mixture of C: H: O of around 1: 2000: 1.7, at 10 −4 atm and around 400 Kelvin, even amino acids , purines , pyrimidines and the like can be formed - simulated in earthly laboratories ).
Even better conditions for the construction naturally prevail on the planetary surfaces protected by atmospheres. From an astrochemical point of view, it is highly probable that there are a number of places in the depths of space for the formation of biochemical molecules, indeed for the formation of life itself, and have probably existed for a long time (the problem of establishing contacts with extraterrestrial civilizations, however, lies not in the missing, irrefutable evidence of their existence - but in the seemingly unbridgeable, great distance between them).
Earthly matter - viewed cosmochemically
The frequency distribution of the elements in the cosmos as a whole can change very locally. One such process that changes this average distribution is gravitation. It is the force by which the solar system emerged from a rotating cloud of gas and dust ( nebular hypothesis by Pierre-Simon Laplace , originally formulated by Immanuel Kant in 1755 in his work General Natural History and Theory of Heaven , collectively Kant-Laplace- Theory ).
Formation of earth, planetary and solar systems
According to contemporary views, about 4.6 billion years ago, instead of our solar system, an extensive cloud of matter moved around the center of the galaxy. The cloud consisted of more than 99% of the gases hydrogen and helium as well as a small proportion of only micrometer-sized dust particles , which were composed of heavier elements and compounds such as water , carbon monoxide , carbon dioxide , other carbon compounds , ammonia and silicon compounds. The hydrogen and most of the helium had already been created during the Big Bang . The heavier elements and compounds were created inside stars and released when they exploded. Parts of the matter cloud contracted as a result of its own gravity and condensed. This could have been triggered by the explosion of a relatively nearby supernova , the pressure waves of which migrated through the cloud. These densifications led to the formation of probably several hundred or even thousands of stars in a star cluster , which probably disintegrated into free single or double stars after a few hundred million years.
Since the angular momentum must be maintained during the contraction , an already minimal rotation of the collapsing cloud has increased, similar to how a figure skater achieved a fast rotation by putting on her arms. The resulting centrifugal forces acting outwards caused the cloud to develop into a rotating disk in the outer areas.
Almost all of the matter in the cloud fell into the center, however, forming a protostar that continued to collapse until the nuclear fusion process was ignited: our sun was formed. In the remaining protoplanetary disk , the clumping of dust particles (coagulation) led to the formation of planetesimals. Planetesimals are the precursors and building blocks of planets . They are formed through accretion , a process by which microscopic dust particles from a presolar nebula (the precursor to a solar system ) aggregate into larger particles . If such particles collide at low speed , they stick together due to chemical bonds or surface adhesion.
These soon-to-be-kilometer-sized structures possessed enough mass to unite with other planetesimals to form larger objects due to their gravity. The heaviest objects exerted the greatest gravitational forces, attracted matter from a large radius and could thus grow even faster. The “Protojupiter” finally disturbed other planetesimals with its gravitational field and influenced their growth. Apparently, it also prevented the formation of a larger body between the orbits of Mars and Jupiter, which led to the formation of the asteroid belt. In just 100,000 years, the planetesimals of the early solar system could evolve into planetary bodies the size of the Earth's moon or the planet Mars .
Similar processes in the formation of the planetary system must have taken place elsewhere in space. Many exoplanets and planemos have been discovered in recent years. Here, too, the volatile and less volatile elements condensed into chemical compounds in space, and many astronomers and astrochemists assume that planemos exist that move in moderate temperature zones around their respective fixed stars. Thus it is conceivable that an extraterrestrial chemistry also produced life in the unreachable depths of the cosmos.
The chemistry of the solar system
Solar systems are created by the gravitational contraction of disc-shaped, rotating disks of matter. Thermodynamic calculations in relation to this disc, which cools ever faster and lighter from the center, show that condensation occurs when the partial pressure p (i) and the vapor pressure of a substance i become equal. The partial pressure of an element in the cosmic gas is mathematically equal to the product of its frequency A (i) relative to that of the hydrogen A (H2), multiplied by the total pressure Pg of the gas: p (i) = A (i) / A (H2) x Pg .
If the vapor pressure p of an element appears as a function of the temperature according to Clausius-Clapeyron, then when the partial and vapor pressure of the element is equated, its condensation temperature can be calculated (i.e.: log po = -A / T + B, -where the factor A is the is the enthalpy of evaporation divided by 2.3 x R and B is the entropy of evaporation divided by 2.3 x R with R as the general gas constant).
Here is the result of this calculation, beginning with the highest condensation temperature with steadily progressing cooling: the element osmium already condenses at temperatures around 1860 K, around 1780 zirconium IV oxide and rhenium, around 1700 aluminum oxide, around 1560–1500 calcium titanate (perovskite) as well as gehlenite (a silicate) and rare earths (U, Th, Ta, Nb), around 1390 the ferromagnetic metals (Fe, Ni, Co), in 1370–1250 magnesium silicates and the metals copper, germanium and gallium (in alloy with Fe ) as well as alkali silicates (with CaAl2Si2O8), at 1100-700 K silver (Ag) and below 750 K there is oxidation of condensed iron (to minerals like FeO + FeS).

In somewhat cooler regions further away from the primordial sun, lead, bismuth, indium and thallium condensed at 600-400 K; hydrated silicates crystallized from 350 K and - in the region of the gas giants farther from the sun - water ice (then NH 4 SH, at <140 Kelvin, at <100 K solid ammonia hydrate, at <60 Kelvin methane hydrate and only at low temperatures of <20 Kelvin also solid methane and argon).
During the condensation, fractionation occurs, ie when "freezing out" the substances are sorted according to their density, both within small clumps (chondrules, meteorites) and on a large scale (planetesimals: outside later gas giants like Jupiter and Saturn or "snow clumps") like comets, planets more compact inside like Mercury and Venus). The substances also separate in the glowing liquid planet (sinking of the metals into the core, subsequent cooling of the outer silicate crusts).
This explains the current chemical structure of our solar system from the inner rock planets to the outer, cool gas giants to the most distant objects in the Kuiper belt and in the Oort cloud from cosmochemistry.
According to Oort (1950), the comets come from a reservoir of 0.1-0.01 solar masses at a distance of about 50,000 AU. Arriving 1 AU close to the sun, they develop halos and - in the immediate vicinity of the nucleus - comas with expansion speeds of 500 m / s, the gas density of which ranges from 10 14 molecules / cm 3 close to the nucleus to 100 molecules / cm 3 outside . The gas and dust particles are driven away by the solar wind and form the comet's ion and dust tail.
The emission spectra of the coma have been examined in detail, and the Giotto probe was able to fly through the coma of the Halley comet unharmed. At a distance of <2 AU, the cometary coma shows bands of cyan, OH radicals, neutral oxygen, sodium and - closer than 1 AU to the core - lines of the elements Cr, Mn, Fe, Co, Ni, Cu, K and Ca , the radicals NH and CH as well as the gases methyl cyan, HCN and water. The CN radicals could have been created by photodissociation of methyl cyan, the NH * radicals by photolysis of hydrazine or of amines such as methylamine, of isocyanic acid (HNCO), methyleneimine H 2 C = NH or of formamide. Radicals like C2 and C3 have also been discovered. They probably come from acetylene and diazomethylacetylene. Ionized molecules of carbon monoxide, carbon dioxide, water and nitrogen were also detectable.
With regard to silicate-like materials, it can be calculated that cometary dust grains are lost due to the radiation pressure of the sun and that their diameter must therefore be less than 10 −6 cm. At first, comets were thought to be “dirty balls of ice”. However, when extremely little methane was found in Comet Kohoutek, it was decided that they could not have originated from cooling, solar gas, but rather contain main components such as water, carbon monoxide, nitrogen as well as hydrogen cyanide, methyl cyanide and dust - materials made from inaccessible Deeps of space, the interstellar gas.
Earthly matter
On earth there is a different distribution of elements than in comets, on distant gas planets or even in the cosmos in general. Looking at the earth's crust , bound oxygen (O) dominates with a mass fraction of 49.2%, followed by silicon (Si, 25.7%), aluminum (Al 7.5%), iron (Fe 4.7%) , Calcium (Ca 3.4%), sodium (Na 2.6%), potassium (K 2.4%), magnesium (Mg 1.9%), hydrogen (H 0.9%) and titanium (Ti 0 , 6%). all other elements only have a mass fraction of less than 0.2%.
If you look at the whole earth and its core, the picture is somewhat different. The most common elements in the entire earth are iron (Fe, 35%) before oxygen (30%), silicon (15%) and magnesium (13%), followed by nickel , sulfur , calcium , aluminum and others (each below three percent) .
Our biomass - cosmochemically analyzed
Humans are made up differently than space and earth: they mainly consist of hydrogen, oxygen, carbon and nitrogen, together with sodium , magnesium , potassium , calcium , phosphorus and sulfur , these elements make up 99.996% of all atoms in a human body (The first systematic studies of the element abundance come from Victor Moritz Goldschmidt , after him the graphic representation of the element abundance is called Goldschmidt diagram ).
Cosmochemists assume that initially only few or no light elements (including carbon, nitrogen and oxygen) were “left” when the solar system was formed on earth and on all other planets close to the sun because of the relatively high temperatures and the effects of the solar wind . According to this theory, all these elements, which today make up the main part of the biosphere, would have been delivered from the outer areas of the solar system by comet impacts for some time after the protoplanets had cooled down a little. Since large impact events from celestial bodies were repeated over and over again during the first several hundred million years after the formation of the solar system, living systems that were already developing during these times would have been repeatedly destroyed by global sterilizations caused by large collisions. The development of life could only start after liquid water was able to persist at least in the deepest parts of the ocean.
The slow cooling of the earth, the resulting volcanism (outgassing from the earth's interior) and the global distribution of the matter of impacted comets led to the establishment of an atmosphere. The main components to be expected in this are water vapor (up to 80%), carbon dioxide (up to 20%), hydrogen sulfide (up to seven percent), ammonia and methane.
However, the actual origin of the water is not entirely undisputed. Especially from water, methane and ammonia, under the conditions of the early earth, small organic molecules (acids, alcohols, amino acids) and later organic polymers (polysaccharides, fats, polypeptides) can form, which are not stable in the oxidizing atmosphere.
The high levels of UV radiation caused a photochemical breakdown of the water, methane and ammonia molecules, causing carbon dioxide and nitrogen to accumulate. Most of the light gases such as hydrogen or helium evaporated into space, while large amounts of carbon dioxide were dissolved in the oceans, acidifying their water and reducing the pH to around 4. The inert and sparingly soluble nitrogen N 2 remained unchanged, accumulated over time and formed the main component of the atmosphere about 3.4 billion years ago.
The precipitation of carbon dioxide with metal ions as carbonates and the later development of organisms that assimilated carbon dioxide led to a reduction in the CO 2 concentration and a re-increase in the pH values of the water bodies. The oxygen O 2 only plays the main role in the further development towards our present atmosphere. It was formed by the appearance of living things with oxygenic photosynthesis for about 3.5 billion years; presumably they were cyanobacteria or cyanobacteria-like prokaryotes.
Biomolecules
The chemical evolution presumably proceeded in such a way that complex, organic molecules - carbon compounds - were formed from the accumulated elements on the developing earth. The prebiotic formation of the complex organic molecules can be divided into three steps:
- Formation of simple organic molecules ( alcohols , carboxylic acids , heterocycles such as purines and pyrimidines ) from inorganic substances.
- Formation of the basic building blocks ( simple sugars , amino acids , pyrroles , fatty acids , nucleotides ) of complex organic molecules from simple organic molecules.
- Creation of the complex organic molecules from the basic building blocks.
The elemental analysis of these molecules leads to the question of which inorganic compounds were necessary for their formation. These had to be present in the reducing primordial atmosphere of the earth - in the distribution and under the reaction conditions that chemically enabled the emergence of the first living beings.
A particularly intensive form of involvement of minerals and rocks in the prebiotic synthesis of organic molecules must have taken place on the surface of iron sulfide minerals. The scenario for the early chemical evolution of life was developed by Günter Wächtershäuser since the early 1980s .
According to this, life on earth would have originated on the surface of iron-sulfur minerals (the iron-sulfur world ESW), i.e. on sulphides that are still formed today through geological processes in deep-sea volcanoes and were still essential in the early days of the earth must have occurred more frequently (» black smokers «).
After all, ribonucleic acid (RNA) forms a molecule essential for the creation of life. The RNA world hypothesis was first proposed by Walter Gilbert in 1986 . This assumption can be derived from the ability of RNA to store, transfer and reproduce genetic information and from its ability to catalyze reactions as ribozymes. In an evolutionary environment, those RNA molecules would occur more frequently that preferentially reproduce themselves. Due to various properties, RNA is believed to be older than DNA.
See also
- geochemistry
- IR spectroscopy
- Primordial nucleosynthesis
- chemical evolution
- Astrobiology
- Astrochemistry
literature
- EM Burbidge, GR Burbidge, WA Fowler, F. Hoyle: Synthesis of the Elements in Stars. In: Rev. Mod. Phys. 29. 1957, p. 547 ( direct PDF download link ).
- Paola Caselli, Cecilia Ceccarelli: Our astrochemical heritage , in: The Astronomy and Astrophysics Review, October 2012, 20:56, 1–68, also online at Arxiv.org, English, PDF
- CE Rolfs, WS Rodney: Cauldrons in the Cosmos. Univ. of Chicago Press, 1988
- Heinz Oberhummer : Cores and Stars. Barth, Leipzig 1993, ISBN 3-335-00319-5
- Wolfgang Kiesl: Cosmochemistry. Springer, Vienna 1979, ISBN 3-211-81527-9
- Charles R. Cowley: An introduction to cosmochemistry. Cambridge Univ. Press, Cambridge 1995, ISBN 0-521-41538-1
- César Esteban: Cosmochemistry - the melting pot of the elements. Cambridge University Press, Cambridge 2004, ISBN 0-521-82768-X
- Andrew M. Shaw: Astrochemistry - from astronomy to astrobiology. Wiley & Sons, Chichester 2006, ISBN 0-470-09136-3
- DD Clayton: Handbook of isotopes in the cosmos. Cambridge Univ. Press, Cambridge 2003, ISBN 0-521-82381-1
- Thomas Henning : Astromineralogy. Springer, Berlin 2003, ISBN 3-540-44323-1
Web links
- Astrochemistry.eu, EU online forum in the field of observational and laboratory astrochemistry and physics
- Research group cosmochemistry, University of Cologne
- The astrochemist
- Astrochemistry Laboratory at the NASA Goddard Space Flight Center
- The Big Picture-Cosmochemistry (PDF; 2.4 MB) William M. White, Cornell University 2005, Geochemistry (pdf, 58 p., Accessed January 1, 2011; 4 MB)