Airgel
Aerogels [ aeːroˌɡeːl ] are highly porous solids , in which up to 99.98% of the volume consisting of pores. There are several types of aerogels, the most common of which are silicate-based ones. Other materials, for example based on plastic or carbon , are used in special cases. In principle, all metal oxides , polymers and some other substances can be used as a starting point for airgel synthesis using a sol-gel process .
Properties and structure
Aerogels have a strongly dendritic structure, i.e. a branching of particle chains with a large number of gaps in the form of open pores. These chains have contact points, so that ultimately the image of a stable, spongy network results. Its aggregates have a fractal dimension , so they are to a certain extent self-similar .
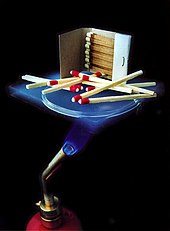
The pore size is in the nanometer range and the inner surfaces can be exceptionally large with up to 1000 m² / g. This allows aerogels u. a. can be used as thermal insulation or filter material. It is also possible to store biologically active molecules, proteins or even whole cells. Aerogels hold 14 entries in the Guinness Book of Records for material properties, including “best insulator” and “lightest solid”. In 2012, Aerographite with 99.99% air and 0.01% graphitic carbon was developed as the record holder in the category “solids with the lowest density” . It is pitch black, stable, electrically conductive, malleable and opaque.
Since silicate aerogels in particular have been studied comparatively well in their diversity, it is possible to give quite precise information for this airgel group. Their properties are qualitatively as well as to a large extent also quantitatively quite similar to those of the other aerogels with, however, some specific peculiarities. The exact material properties can - depending on the starting material and manufacturing process - differ greatly from one another; they can therefore be tailored to the desired use. The term silicate airgel, however, refers to the structure and less to the chemical composition of the material. The latter corresponds roughly to SiO (OH) y (OR) z , with y and z as parameters depending on the manufacturing process.
The high optical transparency, together with a refractive index of around 1.007 to 1.24 and a typical value of 1.02, also make aerogels interesting from an optical point of view. A silicate airgel appears milky-blue against a dark background because the silicon dioxide scatters the shorter wavelengths (that is, the blue components of white light ) more than the longer-wave radiation. This effect can also be observed in the form of Rayleigh scattering in daylight in the earth's atmosphere . Due to this property, silicate aerogels appear semi-transparent to transparent (see photos on the right) and are therefore also known as “frozen smoke” or “blue smoke”. Despite its transparent appearance, the airgel feels like hard plastic foam.
The individual particles of the silicate aerogels are around one to ten nanometers in size and the distance between the chains is around 10 to 100 nm. The cylindrical mesopores are quite accessible and, by definition, have a diameter of 2 nm to 50 nm, whereby the porosity ( the volume fraction of the air pores) is in the range from 80 to 99.8%. The bulk density is therefore in the range of 0.16 ( aerograph ) to 500 mg / cm³ with a typical value of 100 mg / cm³ (air in the pores not included in the mass), whereas the true density (base material without pores) is 1 , 7 to 2.1 g / cm³. Accordingly, silicate aerogels have a very high specific surface area of 100 to 1,600 m² / g and a typical value of 600 m² / g .
The thermal conductivity in air at 300 Kelvin is extremely low at 0.017 to 0.021 W / (m · K) and a typical value of 0.02 W / (m · K), which gives the aerogels a high temperature stability even under extreme conditions the best thermal insulation materials to date. Other sources state up to 0.03 W / (m · K).
There is also a very high density of states , which is associated with a greatly increased specific heat capacity at low temperatures.
Silicate aerogels cannot be wetted or chemically attacked by liquid metals, so they are chemically inert to them . Their melting point is around 1,200 ° C. They are also non-flammable and non-toxic. However, they absorb humidity and tend to crack when drying.
Another property is the low speed of sound with 20 to 800 m / s and a typical value of 100 m / s and, associated with this, also low acoustic field impedance within aerogels.
The modulus of elasticity is in a range from 0.002 to 100 MPa, with a typical value of 1 MPa.
A phenomenon that could be observed with aerogels is that they can sound in the range that can be heard by humans, i.e. represent resonance bodies. The frequency depends on the type of excitation. This effect is due to acoustic shear waves that are excited when the gel is hit.
Manufacturing

Aerogels are made by drying a gel made of a gelatinous substance, usually silica , under extreme conditions. The first synthesis of silicate aerogels was achieved by Samuel Stephens Kistler in 1931/32. He was the first to develop a method for drying gels without them shrinking.
Silicate airgel according to Kistler
Kistler used sodium silicate , which he mixed with water to create a solution ( water glass ). After the addition of a precipitating - reagent acting hydrochloric acid fell with time silica of ( precipitation reaction through), which due to Brownian motion distributed uncoordinated in the solution and thereby also collided.
Or:
Due to the gradual adhesion, these particles aggregated over time and within about a day a gel with a network-like structure resulted. From this, the sodium chloride and the excess hydrochloric acid were rinsed out with water (Aquagel), and alcohol was added (Alkogel). This step is necessary because the water would otherwise destroy the gel structure again in the further course of the process. If the alcohol evaporates slowly, menisci form due to the surface forces that act, which “dig” into the gel and create a duct-like structure in it. Associated with this would be a shrinkage of the gel and, as a result, a porous structure with only about 50% pores, which, however, had to be avoided. Kistler therefore used an autoclave for drying and increased the temperature and pressure above the critical point of alcohol, creating a supercritical fluid. This procedure is known as supercritical drying . The phase boundary between gas and liquid was thus eliminated; Surface forces, which in the other case would have led to the formation of menisci, no longer existed. The supercritical fluid was then vented from the autoclave, drying the product and eventually becoming an airgel. The airgel retained the size and shape of the original gel, with the silicate aerogels manufactured by Kistler having a density of around 30 to 300 kg / m³ and a porosity in the range between 86 and 98%. The Kistler manufacturing method, however, had the disadvantage of being long and complex, which particularly concerned the solvent exchange before the alcohol evaporated.
Teichner method - the sol-gel process
Stanislas Teichner tried to reproduce Kistler's method at the University of Lyon in the 1960s , but it took him weeks to produce smaller airgel samples. As an alternative, he developed the sol-gel process, which is now used as the standard process, in 1968, which was further improved in 1986. The starting material here is the toxic tetramethylorthosilicate (TMOS) which, according to the reaction equation below, slowly hydrolyzes with a defined amount of water after the addition of a catalyst to orthosilicic acid and methanol .
Water is then split off from the silica and SiO 2 tetrahedra are formed. These then network to form a gel. The resulting alcogel is again dried in the same way as the Kistler process, with the methanol having critical values of 239.4 ° C. and 80.9 bar. The properties of the airgel thus formed, in particular structure and density, can be controlled through the choice of the catalyst, the pH value or the quantitative ratio of the substances used, in particular the methanol. The process is used today at DESY and in Lund .
Other procedures
In another process, a research group led by Arlon Hunt at the University of California at Berkeley is producing pieces of airgel from tetraethylorthosilicate (TEOS) instead of the toxic TMOS . In addition, the combustible ethanol is replaced by carbon dioxide , which is very time-consuming. One advantage is the relatively low critical temperature of the carbon dioxide at 31 ° C , which makes the drying process much easier.
Another method is used at BASF in Ludwigshafen am Rhein , where in particular airgel beads (granules) with a diameter of around one to six millimeters and a density of around 200 kg / m 3 are produced. Sulfuric acid and sodium silicate are reacted by spraying them onto a flask with a mixing nozzle . This leads to the formation of alkali salts , which have to be washed out by post-processing. The advantage of this process lies in the comparatively lower costs, the disadvantage can be seen in the poorer, in particular optical properties of the granulate.
Carbon aerogels (CRF) are mainly produced by the pyrolysis of resorcinol - formaldehyde aerogels (RF). In the production of resorcinol-formaldehyde aerogels, cheaper air drying can be used instead of supercritical drying.
Applications
Since the refractive index of the aerogels is in a range that cannot be reached by gases, liquids or conventional solids, they play an important role as so-called radiator material for Cherenkov detectors ; Carbon aerogels also because of their high electrical conductivity and stability in material research for electrode material in primary and fuel cells , vehicle catalysts and supercapacitors .
Storage medium for gases and solids
Because of their high porosity , aerogels were initially developed with the aim of obtaining storage options for gases and solids. In the 1960s, aerogels were investigated for their suitability as storage media for liquid rocket fuel.
Filtering
Due to their fine structure, aerogels can be used as a collection matrix for the smallest dust particles. They were therefore used on board the “comet dust space probe ” Stardust . The trapped dust particles and molecules are slowed down slowly in the airgel so that they are not thermally destroyed. So it succeeded u. a. also the first time to bring undamaged material from a comet ( Wild 2 ) to earth.
Thermal insulation
Silicate aerogels in particular have a very low thermal conductivity and are therefore often used as insulation for special applications (e.g. as transparent thermal insulation ); A corresponding special plaster with added airgel granulate has been sold in Switzerland since the beginning of 2013. Airgel granules are also used as blown insulation. Due to the small grain size and high insulating effect, it is particularly suitable for subsequent core insulation of double-shell masonry with only a narrow gap.
Cosmetics and hair care
Fine, hydrophobic airgel particles made of silica silylates are used, among other things, as setting powder in cosmetics and volume and styling powder in hair care.
pharmacy
In the pharmaceutical sector, silicon dioxide airgel is used as a drying and solvent and as a carrier.
literature
- SS Kistler: Coherent expanded aerogels and jellies . In: Nature. 127, 1931, ISSN 0028-0836 , pp. 741ff., Doi : 10.1038 / 127741a0 .
- SS Kistler: Coherent expanded aerogels . In: Journal of Physical Chemistry. 36, No. 1, 1931, ISSN 1520-6106 , pp. 52-64., Doi : 10.1021 / j150331a003 .
- Jochen Fricke: Aerogels . In: Physics in Our Time. 17, No. 4, 1986, ISSN 0031-9252 , pp. 101-106.
- Nicola Hüsing , Ulrich Schubert : Aerogels - airy materials: chemistry, structure and properties . In: Angewandte Chemie . 110, No. 1-2, 1998, ISSN 0044-8249 , pp. 22-47.
- Matthias Koebel, Arnaud Rigacci, Patrick Achard: Airgel-based thermal superinsulation: an overview . In: Journal of Sol-Gel Science and Technology . 63, No. 3, 2012, pp. 315–339. doi : 10.1007 / s10971-012-2792-9 .
Web links
- Properties and possible uses of airgel windows in comparison with conventional and evacuated windows. PhD thesis, Georges Reber, 1991 (PDF file; 1.67 MB)
- Lawrence Berkeley National Laboratory website on the thermal properties of silicate aerogels
- Lawrence Berkeley National Laboratory: Evolution of Aerogels
Individual evidence
- ↑ Matthias Mecklenburg, Arnim Schuchardt, Yogendra Kumar Mishra, Sören Kaps, Rainer Adelung, Andriy Lotnyk, Lorenz Kienle and Karl Schulte: Aerographite: Ultra Lightweight, Flexible Nanowall, Carbon Microtube Material with Outstanding Mechanical Performance, Advanced Materials, Volume 24, Issue 26 , 2012, doi : 10.1002 / adma.201200491
- ^ "Thermal conductivity" in Lide, DR, ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5 . Section 12, p. 227.
- ↑ SS Kistler: Coherent expanded aerogels and jellies . In: Nature. 127, 1931, ISSN 0028-0836 , pp. 741ff., Doi : 10.1038 / 127741a0 .
- ^ SS Kistler: Coherent expanded aerogels . In: Journal of Physical Chemistry. 36, No. 1, 1932, ISSN 1520-6106 , pp. 52-64.
- ↑ Presentation of the Federal Materials Testing and Research Institute .
- ↑ Core insulation: This is how you insulate double-shell masonry . energie-experten.org. Accessed January 31, 2020.
- ↑ Airgel granules P400 . InnoDämm. Accessed January 31, 2020.