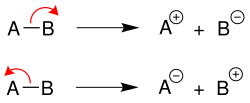
Heterolytic cleavage
In the case of heterolytic cleavage , the binding electrons remain on a binding partner, thus creating a cation and an anion each . The more electronegative element [in example B (above) or A (below)] takes on the negative charge:
The heterolytic cleavage is often only a borderline case in organic chemistry, since mostly no isolated ions occur, but the bonds are only polarized ; ie the binding partners have a partial charge δ + or δ−. In bromomethane , for example, the C – Br bond is polarized, the carbon atom has a positive partial charge (δ +), the bromine atom a negative partial charge (δ−):
The heterolysis of bromomethane is of secondary importance because it is thermodynamically unfavorable:
The methyl cation has only a low stability.
The heterolysis of tert-butyl bromide is much easier , since the tertiary carbenium ion formed is more stable than the primary methyl carbenium ion, which can be formed from bromomethane:
See also
Web links
Individual evidence
- ↑ Otto-Albrecht Neumüller (Ed.): Römpps Chemie-Lexikon. Volume 3: H-L. 8th revised and expanded edition. Franckh'sche Verlagshandlung, Stuttgart 1983, ISBN 3-440-04513-7 , p. 1693.