Proteasome
| Proteasome | ||
|---|---|---|
| Enzyme classification | ||
| EC, category | 3.4.25.1 , peptidase | |
| MEROPS | T1 | |
| Response type | Proteolysis | |
| Substrate | ubiquitin (yl) ated proteins | |
The term proteasome generally refers to a protein complex that occurs in archaea and some bacteria and is present in eukaryotes in both the nucleus and the cytoplasm. In particular, proteasomes differ in their composition between different taxa . In mammals, proteasomes also exist in various forms within an organism, e.g. B. as a free 20 S proteasome or in combination with one or more regulatory particles (e.g. as a 26S proteasome). Proteasomes play an essential role in the controlled breakdown of proteins in the cell and are therefore an integral part of protein quality control in cells. Proteins intended for degradation are unfolded, introduced into the proteasome and cut into shorter [peptide] s by the catalytically active subunits of the proteasome. Proteasomes are therefore multicatalytic proteases.
structure
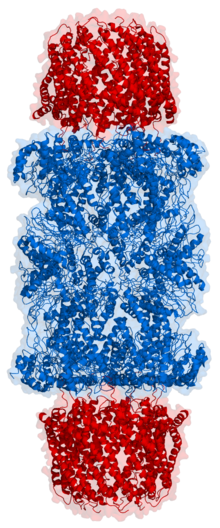
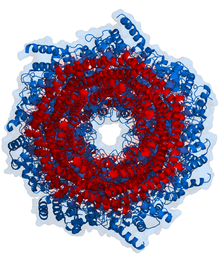
The eukaryotic proteasome consists of the 20S core particle, in which the catalytic protease activities are located, and possibly other regulatory protein complexes that can be bound to one or both ends of the 20S proteasome. Of particular importance is the 19S regulator (also PA700), which plays a central role in the breakdown of ubiquitinated protein substrates. 20S proteasomes in combination with one or two 19S regulators are called 26S proteasomes. The further distinction between 26S (20S + 1 x 19S) and 30S (20S + 2 x 19S) is inconsistent in the literature. In addition to the 19S regulator, there are other regulators such as B. the 11S regulator (PA28αβ or PA28γ) or the regulator PA200 located in the cell nucleus. Proteasomes can also consist of the 20S proteasome with two different regulators, e.g. B. a 19S regulator at one end and a PA28αβ regulator at the other end. These structures are called hybrid proteasomes. The 20S proteasome forms a cylindrical structure made up of four rings of seven subunits each. In the middle of the two α-rings there is a pore (English: gate ) that regulates the entry of substrates into the 20S proteasome. The size of the opening is primarily regulated by the bound regulator complex. The “ gate-opening ” function of the regulator was first described for 20S proteasomes of yeast in combination with PA26 regulators from the protozoon Trypanosoma bruzei . In addition, an allosteric regulation between the catalytically active subunits of the 20S proteasome and the α-ring opening has also been described.
20S proteasome (also 20S nuclear particle)
The 20S proteasome has the shape of a cylinder and acts as a multicatalytic protease . It consists of four rings, each of which is composed of 7 different sub-units. The outer rings consist of α-subunits (α1-α7). In contrast to bacterial and archeae proteasomes, which have several identical catalytic subunits, the inner two rings of the eukaryotic proteasomes each consist of seven β subunits, three of which are catalytically active proteases (β1, β2 and β5). In the middle of the 20S proteasome, an inner chamber is structurally formed in which the cleavage of the substrates takes place. Structurally, there is a further chamber (Antechamber) between the α and β rings. The catalytically active subunits occur in many vertebrates in two (for β1 and β2) or three (for β5) different variants and the composition of the 20S proteasome from the respective variants determines the differentiation of the proteasome as a standard proteasome (also called "constitutive proteasome") ), Immune proteasome or thymoproteasome. In standard proteasomes the subunits β1, β2 and β5 are referred to as standard proteasome subunits (also constitutive subunits). Each of these three subunits has a slightly different proteolytic activity: β1 cleaves the peptide chain of the unfolded protein according to acidic amino acids ( caspase- like activity), β2 cleaves according to basic amino acids ( trypsin- like activity) and β5 splits according to hydrophobic amino acids ( chymotrypsin- like activity) ). The structure of the 20S proteasome could be determined with high resolution by X-ray structure analysis.
19S regulator
In eukaryotes, the 19S complexes sit similar to a cap on one or both openings of the 20S complex. They regulate the access of substrates to the 20S complex by unfolding substrate proteins and regulating the size of the α-ring pore in the 20S complex. The 19S regulator consists of Rpn and Rpt proteins. The different Rpn subunits (regulatory particle non-ATPases) are responsible for the binding of ubiquitinated substrates to the proteasome complex. They regulate the development of the protein destined for degradation, recruit other factors to the proteasome (e.g. the de-ubiquitinating enzyme USP14) or are involved in de-ubiquitination themselves (Rpn11). The six Rpt subunits (regulatory particle ATPases) play a key role in the transfer of the substrate protein into the 20S proteasome. Depending on adenosine triphosphate (ATP) and the hydrolysis of ATP to ADP, the Rpt ring goes through a complex formation cycle through which the substrates are translocated into the 20S proteasome for degradation. Due to its dynamic nature, the structure of the 19S regulator could only be elucidated more recently with the development of cryo-electron microscopy.
Discovery of the proteasome
The discovery of the proteasome goes back to the decade of the 1980s. It was originally described independently under several different names, including the term multicatalytic protease complex , and finally in the late 1980s under the term proteasome , which should indicate the role of this complex in the breakdown of proteins. In the early 1990s, the first genes ( Psmb8 / LMP7 and Psmb9 / LMP2) of the subunits of the so-called immune proteasome were discovered, the position of which lies in the gene cluster of the main histocompatibility complex . In 1996 the third subunit ( Psmb10 / MECL-1) of the immune proteasome was discovered and finally in 2007 another alternative subunit ( Psmb11 / β5t), which occurs exclusively in the thymus. Immune proteasomes and thymoproteasomes have evolved through gene duplication and occur in the vertebrates of the superclass jaw-mouths , but apparently with the exception of birds.
Immune proteasome
The catalytic subunits β1, β2 and β5 are also referred to as constitutive subunits and the complete 20S proteasome with these subunits as the constitutive proteasome or standard proteasome . This more detailed specification is important for the special role of the proteasome in an immune response . In the course of this, cells in places of invading pathogens (viruses, bacteria, parasites) are exposed to the cytokine interferon-γ by cells of the immune system . As a result, the cells change the expression of various genes, which supports the immune response. Instead of the three proteasome subunits β1, β2 and β5, the subunits low molecular mass protein 7 (LMP7, also β5i), multicatalytic endopeptidase complex like 1 (MECL-1, also β2i) and LMP2 (also β1i) are incorporated into the 20S nucleus -Particles incorporated. The proteasome assembled in this way is therefore referred to as an "immune proteasome" . The immune proteasome has numerous functions for the immune response, for example a stronger presentation of antigens via MHC class I compared to the standard proteasome. In addition, other functions are assigned to the immune proteasome that are independent of the antigen presentation , such as. B. an influence on the survival of T cells during a virus infection or an influence on the functional polarization of T helper cells . A special role of the immune proteasome for protein homeostasis in the presence of cellular stress under the influence of inflammatory cytokines such as interferon-γ was found, as well as a possible, special role of the immune proteasome for the NF-κB signaling pathway. However, these functions are discussed controversially in the primary literature and have therefore not yet been conclusively explained. In many cells of the immune system itself, the immune proteasome is permanently formed alongside the standard proteasome. A particularly large amount of immune proteasomes is found in T and B lymphocytes , which almost exclusively contain immune proteasomes.
Thymoproteasome
In addition to the constitutive proteasome and the immune proteasome, there is a third class of proteasome that occurs exclusively in the cortical epithelial cells of the thymus . Here the subunit β5 is replaced by the alternative subunit β5t ("t" for thymoproteasome). The cortical epithelial cells of the thymus play an important role in the process of positive selection during the development of T lymphocytes . If all alternative subunits of the 20S proteasome (β5i, β2i, β1i and β5t) are missing in mice, the mice show a severe defect in the maturation of T cells, since most of these are lost during the negative selection. These experiments provided evidence for the so-called peptide switching model. It says that the cortical thymus epithelial cells generate different peptides through thymo- and immune proteasomes than the medullary thymus epithelial cells and the dendritic cells in the negative selection, so that a successful maturation of the CD8- positive T cells can take place.
Target proteins for degradation by the proteasome
A central process for the regulated breakdown of proteins is what is known as ubiquitination. Proteins that are to be broken down are marked in a multi-step enzymatic process by ubiquitin protein ligases with a poly ubiquitin chain, which is recognized by the 19S complexes. Ubiquitin is a small protein with a molecular mass of 8.5 kDa. Before the substrate is introduced into the 20S proteasome, the polyubiquitin chain is broken down into its individual ubiquitin molecules, which can then be reused. In addition to ubiquitin, the labeling for immediate degradation by the proteasome can also be carried out using the ubiquitin-like modifier FAT10. In contrast to ubiquitin, however, FAT10 is broken down with the substrate. In the presence of cellular stress (e.g. heat shock or oxidative stress), proteins are increasingly damaged and have to be broken down in a regulated manner. Oxidatively damaged proteins and proteins with an intrinsically low folding stability can in part also be degraded directly by free 20S proteasomes.
Importance of the proteasome for the cell
The controlled breakdown of proteins is essential for the cell. Metabolic enzymes , transcription factors or proteins that regulate the cell cycle , such as cyclins and CDK inhibitors, are degraded. Defective proteins are also broken down. Proteins are broken down in a regulated manner depending on their half-life and are regenerated if necessary. Some of the peptides formed by the proteasome are smuggled into the endoplasmic reticulum and then bound to the main histocompatibility complex I and presented to the immune system on the surface of the cell (cf. also section Immune proteasome). Most of the peptides formed, however, are quickly broken down into individual amino acids in the cytosol by further peptidases. The amino acids are then available for the synthesis of new proteins. In contrast to carbohydrates and fats, amino acids cannot be stored in separate storage polymers. Amino acids therefore have to be metabolically formed when there is an increased need for protein synthesis, taken up from the cell environment or obtained through the regulated breakdown of other cellular proteins. Therefore protein synthesis and protein breakdown are closely linked through intracellular signaling processes. Insufficient ability of cells to break down damaged proteins has been linked to neurodegenerative diseases in which undegraded protein aggregates inside and outside cells cause damage in the central and / or peripheral nervous system. One example is amyotrophic lateral sclerosis (ALS), which is related to genetic changes that may cause a. aggregation of proteins in nerve cells. Cryo-electron microscopy has shown evidence that in the case of the C9orf72 mutation (a genetic variant associated with ALS) , the resulting protein aggregates cause blockage of the 26S proteasome, thereby disrupting normal protein quality control.
Proteasome inhibitors
Proteasome inhibitors are chemical substances that inhibit the catalytic activity of the 20S proteasome or the 26S proteasome via the 19S regulator. The proteasome inhibitors developed early on included peptide aldehydes such as the inhibitor MG-132, which is still one of the most frequently used proteasome inhibitors for researching proteasome-dependent processes in cells. Sustained inhibition of the proteasome is toxic to cells. If the proteasome is prevented from breaking down proteins, this may result in a. a lack of necessary amino acids in the cell. This is one of the mechanisms by which the cells enter into cell death. Proteasome inhibitors can also control certain signaling pathways such. B. block the NF-κB signal path. Certain anti- apoptotic factors can therefore no longer be formed and the cell is also led to cell death in this way.
Proteasome inhibitors in clinical use and preclinical development
Proteasome inhibitors are used in the clinical treatment of multiple myeloma and mantle cell lymphoma . The first proteasome inhibitor to be clinically approved in 2003 was bortezomib (trade name Velcade®) and treatment options have more recently been complemented by second-generation proteasome inhibitors such as carfilzomib and ixazomib. Due to the side effects of broad-spectrum proteasome inhibitors such as bortezomib, the clinical use of proteasome inhibitors is currently limited to malignant diseases. In addition, there are limitations in clinical use due to the development of resistance. Numerous other proteasome inhibitors, the effect of which is aimed at certain subunits of the various proteasomes, are currently being developed and researched in the preclinical process. This also includes selective immune proteasome inhibitors such as the active ingredients ONX 0914 or KZR-616. Since these active ingredients inhibit the subunits of the immune proteasome in a more targeted manner, there is hope that they can also be used in the future for non-malignant diseases such as B. in autoimmune diseases or for the treatment of inflammatory diseases such as viral myocarditis .
literature
- Pollard TD, Earnshaw WC, Lippincott-Schwartz J. and GT Johnson: Cell Biology . 3. Edition. Elsevier, 2017, ISBN 978-0-323-34126-4 .
- Collins GA and AL Goldberg: The Logic of the 26S Proteasome . In: Cell . tape 169 , no. 5 , 2017, p. 792-806 , doi : 10.1016 / j.cell.2017.04.023 , PMID 28525752 .
- A. Hershko: The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle . In: Cell Death and Differentiation . tape 12 , 2005, p. 1191-1197 , PMID 16094395 .
- Niewerth D., G. Jansen, YG Assaraf, S. Zweegman, GJ Kaspers and J. Cloos .: Molecular basis of resistance to proteasome inhibitors in hematological malignancies. In: Drug Resistance Updates . No. 18 , 2015, p. 18–35 , doi : 10.1016 / j.drup.2014.12.001 , PMID 25670156 .
- Murata S., Y. Takahama, M. Kasahara, and K. Tanaka: The immunoproteasome and thymoproteasome: functions, evolution and human disease. In: Nature Immunology . tape 19 , no. 9 , September 2018, p. 923-931 , doi : 10.1038 / s41590-018-0186-z , PMID 30104634 .
- Beling A. and M. Kespohl: Proteasomal Protein Degradation: Adaptation of Cellular Proteolysis With Impact on Virus — and Cytokine-Mediated Damage of Heart Tissue During Myocarditis . In: Frontiers in Immunology . tape 9 , no. 2620 , November 2018, p. eCollection 2018 , doi : 10.3389 / fimmu.2018.02620 .
- Basler M., S. Mundt, A. Bitzer, C. Schmidt, and M. Groettrup: The immunoproteasome: a novel drug target for autoimmune diseases . In: Clinical and Experimental Rheumatology . tape 33 , 4 Suppl 92, July 2014, p. S74-9 , PMID 26458097 .
- Budenholzer L., Cheng CL, Li Y. and M. Hochstrasser: Proteasome Structure and Assembly. In: Journal of Molecular Biology . tape 429 , no. 22 , November 2017, p. 3500-3524 , PMID 28583440 .
- Limanaqi F., Biagioni F., Gaglione A., Busceti CL and F, Fornai: A Sentinel in the Crosstalk Between the Nervous and Immune System: The (Immuno) -Proteasome. In: Frontiers in Immunology . tape 10 , no. 28 , March 2019, p. eCollection 2019 , PMID 30984192 .
Individual evidence
- ^ Stadtmueller, BM and CP Hill: Proteasome Activators . In: Molecular Cell . tape 41 , no. 1 , 2011, p. 8-19 , doi : 10.1016 / j.molcel.2010.12.020 , PMID 21211719 .
- ^ A b Whitby FG, EI Masters, L. Kramer, JR Knowlton, Y. Yao, CC Wang and CP Hill: Structural basis for the activation of 20S proteasomes by 11S regulators . In: Nature . tape 408 , no. 6808 , p. 115-120 , doi : 10.1038 / 35040607 , PMID 11081519 .
- ↑ Osmulski PA, M. Hochstrasser and M. Gaczynska: A Tetrahedral Transition State at the Active Sites of the 20S Proteasome Is Coupled to Opening of the α-Ring Channel . In: Structure . tape 17 , no. 8 , 2009, p. 1137–1147 , doi : 10.1016 / j.str.2009.06.011 , PMID 19679091 .
- ↑ Wehmer M., T. Rudack, F. Beck, A. Aufderheide, G. Pfeifer, JM Plitzko, F. Foerster, K. Schulten, W. Baumeister and E. Sakata: Structural insights into the functional cycle of the ATPase module of the 26S proteasome . In: Proceedings of the National Academy of Sciences of the United States of America . tape 114 , no. 6 , February 2017, p. 1305-1310 , doi : 10.1073 / pnas.1621129114 , PMID 28115689 .
- ↑ Groll M., L. Ditzel, J. Löwe, D. Stock, M. Bochtler, HD Bartunik and R. Huber: Structure of 20S proteasome from yeast at 2.4Å resolution . In: Nature . tape 386 , no. 6624 , April 1997, p. 463-71 , doi : 10.1038 / 386463a0 , PMID 9087403 .
- ^ Collins GA and AL Goldberg: The Logic of the 26S Proteasome . In: Cell . tape 169 , no. 5 , 2017, p. 792-806 , doi : 10.1016 / j.cell.2017.04.023 , PMID 28525752 .
- ↑ Lasker K., F. Förster, S. Bohn, T. Walzthoeni, E. Villa, P. Unverdorben, F. Beck, R. Aebersold, A. Sali and W. Baumeister: Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach . In: Proceedings of the National Academy of Sciences of the United States of America . tape 109 , no. 5 , January 2012, p. 1380-7 , doi : 10.1073 / pnas.1120559109 , PMID 22307589 .
- ↑ Lander GC, E. Estrin, ME Matyskiela, C. Bashore, E. Nogales and A. Martin: Complete subunit architecture of the proteasome regulatory particle . In: Nature . tape 482 , no. 7384 , February 2012, p. 186-191 , doi : 10.1038 / nature10774 , PMID 22237024 .
- ↑ Orlowski, N., and S. Wilk: A multicatalytical protease complex from pituitary that forms enkephalin and enkephalin containing peptides. In: Biochemical and Biophysical Research Communications . tape 101 , no. 3 , August 1981, p. 814-22 , PMID 7030330 .
- ↑ Arrigo, A.-P., K. Tanaka, AL Goldberg, and WJ Welch: Identity of the 19S “prosome” particle with the large multifunctional protease complex of mammalian cells (the proteasome) . In: Nature . tape 331 , no. 6152 , January 1988, p. 192-194 , doi : 10.1038 / 331192a0 , PMID 3277060 .
- ^ Nandi D., H. Jiang, JJ Monaco: Identification of MECL-1 (LMP-10) as the Third IFN-γ-Inducible Proteasome Subunit . In: Journal of Immunology . tape 156 , no. 7 , April 1996, pp. 2361-4 , PMID 8786291 .
- ↑ Groettrup, M., R. Kraft, S. Kostka, S. Standera, R. Stohwasser, and PM Kloetzel: A third interferon-gamma-induced subunit exchange in the 20S proteasome. tape 26 , no. 4 , April 1996, pp. 863-9 , doi : 10.1002 / eji.1830260421 , PMID 8625980 .
- ↑ Murata, S., K. Sasaki, T. Kishimoto, S.-I. Niwa, H. Hayashi, Y. Takahama, and K. Tanaka: Regulation of CD8 + T cell development by thymus-specific proteasomes. In: Science . tape 316 , no. 5829 , June 2007, p. 1349-53 , doi : 10.1126 / science.1141915 , PMID 17540904 .
- ↑ Groettrup M., CJ Kirk and M. Basler: Proteasomes in immune cells: more than peptide producers? In: Nature Reviews Immunology . tape 10 , no. 1 , January 2010, ISSN 1474-1741 , p. 73-78 , doi : 10.1038 / nri2687 , PMID 20010787 .
- ↑ Seifert U., Bialy LP, Ebstein F., Bech-Otschir D., Voigt A., Schröter F., Prozorovski T., Lange N., Steffen J., Rieger M., Kuckelkorn U., Aktas O., Kloetzel PM and E. Krüger: Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. In: Cell . tape 142 , no. 4 , August 2010, p. 613-24 , doi : 10.1016 / j.cell.2010.07.036 , PMID 20723761 .
- ↑ Beling A. and M. Kespohl: Proteasomal Protein Degradation: Adaptation of Cellular Proteolysis With Impact on Virus– and Cytokine-Mediated Damage of Heart Tissue During Myocarditis . In: Frontiers in Immunology . tape 9 , no. 2620 , November 2018, p. eCollection 2018 , doi : 10.3389 / fimmu.2018.02620 , PMID 30546359 .
- ↑ Nathan JA, Spinnenhirn V., Schmidtke G., Basler M., Groettrup M. and AL Goldberg: Immuno- and constitutive proteasomes do not differ in their abilities to degrade ubiquitinated proteins. In: Cell . tape 152 , no. 5 , February 2013, p. 1184-94 , doi : 10.1016 / j.cell.2013.01.037 , PMID 23452861 .
- ^ Schmidt C., T. Berger, M, Groettrup and M. Basler: Immunoproteasome Inhibition Impairs T and B Cell Activation by Restraining ERK Signaling and Proteostasis . In: Frontiers in Immunology . tape 9 , no. 2386 , October 2018, p. eCollection 2018 , doi : 10.3389 / fimmu.2018.02386 , PMID 30416500 .
- ↑ Kincaid EZ, S. Murata, K. Tanaka, KL Rock: Specialized proteasome subunits have an essential role in the thymic selection of CD8 (+) T cells . In: Nature Immunology . tape 17 , no. 8 , August 2016, p. 938-945 , doi : 10.1038 / ni.3480 , PMID 27294792 , PMC 4955723 (free full text).
- ↑ Aichem A., S. Anders, N. Catone, P. Rößler, S. Stotz, A. Berg, R. Schwab, S. Scheuermann, J. Bialas, MC Schütz-Stoffregen, G. Schmidtke, C. Peter, M. Groettrup and S. Wiesner: The structure of the ubiquitin-like modifier FAT10 reveals an alternative targeting mechanism for proteasomal degradation. In: Nature Communications . tape 9 , no. 1 , August 2018, doi : 10.1038 / s41467-018-05776-3 , PMID 30127417 .
- ↑ Ben-Nissan G. and M. Sharon: Regulating the 20S Proteasome Ubiquitin-Independent Degradation Pathway . In: Biomolecules . tape 4 , no. 3 , September 2014, p. 862-884 , doi : 10.3390 / biom4030862 , PMID 25250704 .
- ↑ Zhang Y., J. Nicholatos, JR Dreier, SJH Ricoult, SB Widenmaier, GS Hotamisligil, DJ Kwiatkowski, BD Manning: Coordinated regulation of protein synthesis and degradation by mTORC1 . In: Nature . tape 513 , no. 7518 , September 2014, p. 440-3 , doi : 10.1038 / nature13492 , PMID 25043031 .
- ↑ Guo Q., C. Lehmer, A.Martínez-Sánchez, T. Rudack, F. Beck, H. Hartmann, M. Pérez-Berlanga, F. Frottin, MS Hipp, FU Hartl, D. Edbauer, W. Baumeister , R.Fernández-Busnadiego: In Situ Structure of Neuronal C9orf72 Poly-GA Aggregates Reveals Proteasome Recruitment . In: Cell . tape 172 , no. 4 , February 2018, p. 696-705.e12 , doi : 10.1016 / j.cell.2017.12.030 , PMID 29398115 .
- ↑ Kisselev AF, WA van der Linden, HS Overkleeft: Proteasome Inhibitors: An Expanding Army Attacking a unique target . In: Chemistry & Biology . tape 19 , no. 1 , 2012, p. 99-115 , doi : 10.1016 / j.chembiol.2012.01.003 , PMID 22284358 .
- ↑ Ito A., R. Takahashi, C. Muira, and Y. Baba: Synthetic Study of Peptide Aldehydes. In: Chemical and Pharmaceutical Bulletin . tape 23 , no. 12 , 1975, p. 3106-3113 , doi : 10.1248 / cpb.23.3106 .
- ↑ Satoshi Tsubuki, S., H. Kawasaki, Y. Saito, N. Miyashita, M. Inomata, and S. Kawashima: Purification and characterization of a Z-Leu-Leu-Leu-MCA degrading protease expected to regulate neurite formation: A novel catalytic activity in proteasome. In: Biochem. Biophys. Res. Commun . tape 196 , no. 3 , November 1993, pp. 1195-201 , doi : 10.1006 / bbrc.1993.2378 , PMID 8250877 .
- ↑ Rock, KL, C. Gramm, L. Rothstein, K. Clark, R. Stein, L. Dick, D. Hwang, and AL Goldberg: Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. tape 78 , no. 5 , September 1994, pp. 761-771 , PMID 8087844 .
- ↑ Suraweera A., C. Münch, A. Hanssum, A. Bertolotti: Failure of Amino Acid Homeostasis Causes Cell Death following Proteasome Inhibition . In: Molecular Cell . tape 48 , no. 2 , 2012, p. 242-253 , doi : 10.1016 / j.molcel.2012.08.003 , PMID 22959274 .
- ↑ HNM Ergin, Q. Huang, J. Qin, HM Amin, RL Martinez, S. Saeed, K.Barton, S. Alkan: Analysis of expression of nuclear factor κB (NF ‐ κB) in multiple myeloma: downregulation of NF‐ κB induces apoptosis . In: British Journal of Hematology . tape 115 , no. 2 , December 2001, p. 279-86 , doi : 10.1046 / j.1365-2141.2001.03102.x , PMID 11703322 .
- ↑ Ma MH, HH Yang, K. Parker, S. Manyak, JM Friedman, C. Altamirano, Z. Wu, MJ Borad, M. Frantzen, E. Roussos, J. Neeser, A.Mikail, J. Adams, N Sjak-Shie, RA Vescio and JR Berenson: The Proteasome Inhibitor PS-341 Markedly Enhances Sensitivity of Multiple Myeloma Tumor Cells to Chemotherapeutic Agents . In: Clinical Cancer Research . tape 9 , no. 3 , March 2003, p. 1136-44 , PMID 12631619 .
- ^ Niewerth D., G. Jansen, YG Assaraf, S. Zweegman, GJ Kaspers and J. Cloos .: Molecular basis of resistance to proteasome inhibitors in hematological malignancies. In: Drug Resistance Updates . No. 18 , 2015, p. 18–35 , doi : 10.1016 / j.drup.2014.12.001 , PMID 25670156 .
- Jump up ↑ Cromm PM and CM Crews: The Proteasome in Modern Drug Discovery: Second Life of a Highly Valuable Drug Target . In: ACS Central Science . tape 3 , no. 8 , 2017, p. 830-838 , doi : 10.1021 / acscentsci.7b00252 , PMID 28852696 .
- ↑ Basler M., MM Lindstrom, JJ LaStant, JM Bradshaw, TD Owens, C. Schmidt, E. Maurits, C. Tsu, HS Overkleeft, CJ Kirk, CL Langrish and M. Groettrup: Co-inhibition of immunoproteasome subunits LMP2 and LMP7 is required to block autoimmunity. In: EMBO reports . October 2018, p. e46512 , doi : 10.15252 / embr.201846512 , PMID 30279279 .
- ↑ Johnson HWB, E. Lowe, JL Anderl, A. Fan, T. Muchamuel, S. Bowers, D. Moebius, C. Kirk and DL McMinn: A required immunoproteasome subunit inhibition profile for anti-inflammatory efficacy and clinical candidate KZR- 616 ((2S, 3R) -N - ((S) -3- (cyclopent-1-en-1-yl) -1 - ((R) -2-methyloxiran-2-yl) -1-oxopropan-2 -yl) -3-hydroxy-3- (4-methoxyphenyl) -2 - ((S) -2- (2-morpholinoacetamido) propanamido) propenamides) . In: Journal of Medicinal Chemistry . October 2018, doi : 10.1021 / acs.jmedchem.8b01201 , PMID 30380863 .
- ^ Althof, N., CC Goetzke, M. Kespohl, K. Voss, A. Heuser, S. Pinkert, Z. Kaya, K. Klingel and A Beling: The immunoproteasome-specific inhibitor ONX 0914 reverses susceptibility to acute viral myocarditis. In: EMBO Molecular Medicine . tape 10 , no. 2 , February 2018, p. 200-218 , doi : 10.15252 / emmm.201708089 , PMID 29295868 .