Ubiquitin
| Ubiquitin | ||
|---|---|---|
|
||
| Ribbon model | ||
| Properties of human protein | ||
| Mass / length primary structure | 8.5 kDa / 76 amino acids | |
| Identifier | ||
| Gene names | RPS27A ; UBA52; UBB; UBC | |
| External IDs | ||
| Occurrence | ||
| Parent taxon | Eukaryotes | |
Ubiquitin is a small protein that can be found in all eukaryotic cells and cell types - i.e. it is ubiquitous in eukaryotes - and is involved in the regulation of various cell processes.
It is enzymatically coupled to other proteins by means of ubiquitin protein ligases , the properties of which are changed by this ubiquitination . Depending on the number and type of ubiquitin bonds, an ubiquitinated target protein can be promoted or hindered in its interaction with other proteins, its activity can be influenced, its localization in the cell changed or its degradation accelerated. Several ubiquitins attached in a chain mark the protein thus poly-ubiquitinated for the degradation in the proteasome in the protein quality control . Ubiquitinations are also important for the regulation of transcription and translation , are involved in signal transduction and endocytosis, are involved in DNA repair and occur in regulated processes of cell cycle , cell differentiation and inflammatory reactions .
Ubiquitination itself is a multi-phase process, the three main steps of which are catalyzed by different enzymes: ubiquitin-activating (E1), ubiquitin-conjugating (E2) and finally ubiquitin ligases (E3), which bind ubiquitin to certain substrate proteins in different ways.
In contrast, there are a number of different desubiquitinating enzymes (DUB), under whose specific action, among other things, attached ubiquitin molecules can be removed again.
Ubiquitination, and ubiquitination called, is a post-translational modification is of proteins. Comparable modifications are couplings ubiquitin-like proteins such as SUMO , Urm1 or Nedd8 corresponding sumoylation , Urmylierung or neddylation mentioned. In addition, some prokaryotes , for example Mycobacterium tuberculosis , know of a protein analogous to ubiquitin , which is called the prokaryotic ubiquitin-like protein (Pup).
Ubiquitin was discovered in 1975 (called ubiquitous immunopoietic polypeptide ) and characterized in more detail in the following years. In the early 1980s, Aaron Ciechanover , Avram Hershko and Irwin Rose were awarded the Nobel Prize in Chemistry for research into the fundamentals of the ubiquitin system .
structure
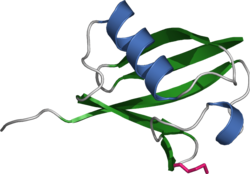
Ubiquitin consists of 76 amino acids and has a molecular mass of 8.5 kDa . Its structure changed little in the course of evolution , so it is highly conserved . The protein in humans and the unicellular yeast Saccharomyces cerevisiae differ in only 3 of the 76 amino acids.
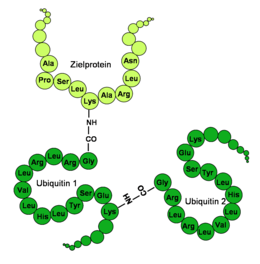
Ubiquitin has a globular shape, only the last four C -terminal amino acids protrude. Important functional amino acids are the C -terminal glycine (G) in the 76th position (G76) and the lysines (K) in the 48th (K48) and 63rd position (K63) of the amino acid sequence . Via the C -terminal carboxyl group on G76, ubiquitin is covalently bound to specific lysines, cysteines, serines, threonines or the N -terminus of the protein to be labeled . Additional ubiquitin molecules can be attached to an already bound ubiquitin via the lysines, so that a ubiquitin chain is formed. Since a ubiquitin contains a total of seven lysines, at least seven different types of compound of a ubiquitin are possible.
The amino acid sequence for human ubiquitin in one letter code - K48, K63 and G76 highlighted in bold:
| N -term MQIFVKTLTGKTITLEVEPSDTIENVKAKIQDKEGIPPDQQRLIFAGKQLEDGRTLSDYNIQKESTLHLVLRLRGG C -term |
Mechanism of Ubiquitination
The process of marking target proteins with ubiquitin is called ubiquitination or ubiquitination . Which process requires - as a sumoylation , Urmylierung or neddylation - a plurality of successive reaction steps , and is of three enzymes catalyze , ubiquitin-protein ligases , which according to the reaction sequence as E1 (activating and modification enzyme), E2 (also modification conjugating enzyme) and E3 (also E3 ligase ) are designated.
In the first step, ubiquitin is bound by a thioester bond between its C -terminal carboxy group (G76) and a cysteine of the E1 enzyme and thus “activated”. This activation is energy dependent; the energy is provided by splitting ATP into AMP and pyrophosphate . There is a specific E1 enzyme for activating the modifier molecule, and in plants there are even two E1 enzymes for ubiquitin.
After ubiquitin has been bound to E1, the ubiquitin is transferred to the enzyme E2. For ubiquitin, more than eleven different E2 enzymes are known in yeast alone, in other organisms their number is even greater (while a specific E2 enzyme each exists for Sumo1 and Nedd8).
In the last step, the ubiquitin is transferred to the target protein by specific E3 ligases. An isopeptide bond is formed between the C -terminal glycine of the ubiquitin and a lysine of the target protein. In contrast to a classic peptide bond , it is not the α-amino residue but the ε-amino residue of lysine that serves as the binding partner. In addition, ubiquitins can also be connected in other ways, and lysine-free proteins have also been found ubiquitinated. The variety of target proteins modified by ubiquitin is reflected in the number of different E3 enzymes. If one takes into account all the enzymes that structurally belong to the three subfamilies of the E3 enzymes (HECT, RING and U-Box), a number between several hundred and one thousand can be assumed for higher organisms.
Types of ubiquitination
Ubiquitins can be bound to the respective target protein in various ways and others can be attached at different points. According to the number of connected ubiquitin molecules, a distinction is made between mono- and oligo-, multi- or poly-ubiquitination, depending on whether there is only one molecule or a few, several or many ubiquitins.
If at least five ubiquitin molecules are linked as a chain to a target protein, it is called poly-ubiquitination. If these molecules are linked to one another at lysine 48 (K48), the target protein is mainly broken down by the proteasome . Connection at lysine 63 (K63) can lead to lysosomal degradation of the protein. Furthermore, it was observed that this modification has an influence on the cellular tolerance of DNA damage , inflammatory immune responses, endocytotic processes and ribosomal protein synthesis.
Mono- and multi-ubiquitinations, on the other hand, affect less the stability of individual proteins than their intracellular distribution and can enable interaction with other proteins. Oligo-ubiquitination, for example, influences the activity of a transcription factor without initiating its degradation.
Examples of ubiquitination
Breakdown of incorrectly folded proteins
The ubiquitin proteasome system plays an important role in the "quality assurance" of intracellularly produced proteins. Proteins should be properly folded during and after their production in order for them to function. The folding of some proteins is as complex and error-prone as in the chloride ion channel CFTR in epithelial cells , in which up to 60–80% of the proteins produced are incorrectly folded. These incorrectly folded proteins are bound by so-called chaperones , enzymes that may promote the correct folding of the protein. In the event of an “irreparable” misfolding, the formation of a protein-chaperone-ubiquitin-E3 ligase complex was observed, which poly-ubiquitinates the misfolded protein and thus enables degradation by the proteasome. In this way, it is ensured that structurally degenerate proteins neither cytosolically nor membrane- associated affect the cell processes.
If, however, in the case of the ion channel CFTR, a mutation occurs in the coding DNA , which is reflected in a mutation of the phenylalanine at position 508 (F508), this leads to poly-ubiquitination and premature degradation of all CFTR proteins produced. The consequence is the clinical picture of cystic fibrosis . Although the mutated ion channel protein cannot function properly, it is broken down prematurely. This example shows that the strict control system of ubiquitin-mediated degradation of structurally incorrect proteins, which actually has a positive effect, can also have a negative effect on the organism.
Regulation of transcription
The first step in protein synthesis is transcription . Here, DNA is transcribed into RNA using an enzyme, RNA polymerase . For the transcription start of the polymerase several are involved in DNA transcription factors required. The accessibility of the DNA for the transcription factors and the polymerase can be regulated by permanently DNA-bound protein complexes, the histones . Histones that are “wrapped” in DNA are called nucleosomes .
The ubiquitin-linking protein Rad6, which regulates the transcription of ARG1 (argininosuccinate synthase gene 1), was discovered in baker's yeast. In the absence of Rad6, the transcription factors and the polymerase can bind to the promoter (a regulatory DNA sequence ) in front of the ARG1 gene and start transcription. In the presence of Rad6, it links a ubiquitin molecule to the lysine K123 of a histone subunit H2B. This leads to modifications of an H3 histone in the neighboring nucleosome: Histone H3 is methylated at the lysines K4 and K49. As a result, the promoter is immobilized so that no transcription factors can bind. As a result of this gene silencing , the ARG1 gene is no longer expressed and the enzyme argininosuccinate synthase is no longer produced in the cell.
In addition, the histone H2A from the fruit fly was the first ubiquitinated protein to be described. In mammals, the ubiquitination state of the histones H2A and H2B became the first marker for transcriptionally active chromatin , the entirety of DNA and its associated proteins.
Ubiquitin as part of signal transduction
Ubiquitin is involved in the intracellular signal transmission of external stimuli, such as in the NF-kB signaling pathway ( engl. Nuclear factor kappa B). This can be activated by the signaling molecule tumor necrosis factor (TNF). If TNF binds to the TNF receptor of the cell membrane, its conformational change recruits the E3 ligase TRAF2 to the intracellular part of the receptor. This poly-ubiquitinates itself and the RIP protein via K63 connections. Various kinases , phosphorylating enzymes, are activated by the ubiquitinated proteins RIP and TRAF2 . The Iκ kinase β ultimately phosphorylates the IκB protein. This now releases the previously bound and inactive NF-κB. NF-κB migrates into the cell nucleus and activates the transcription of certain genes there. IkB, on the other hand, is poly-ubiquitinated via K48 and degraded via the proteasome.
More examples of ubiquitination
- After the end of mitosis , the cyclin involved in the cell cycle is labeled and broken down by ubiquitination.
- In HIV infection, the cell's anti-viral enzymes (ABOBEC3G) are bound by a viral HIV protein (Vif). Vif can also bind parts of the ubiquitination machinery. Vif is thereby ubiquitinated and degraded together with APOBEC3G, whereby the efficiency of the HIV infection is increased.
- Increased mono-ubiquitination occurs in the differentiation of multipotent stem cells .
Diseases
The Angelman Syndrome is a neurological disorder that u itself. a. expresses itself through slowed cognitive and motor development. The most common genetic defect is a 4 million (MBp) base pair deletion on the maternal chromosome 15 gene locus q11-13. However, this region is only active in the hippocampus and in the cerebellum and codes u. a. for the E3 ubiquitin ligase E6-AP. Mice that lack this ligase develop learning deficits, for example in the conditioning of fear. In addition, the long-term neuronal plasticity of the mice is no longer given. These deficits correlate in part with the impairments of patients with Angelman syndrome.
- In the dominantly inherited disease of Hippel-Lindau , a mutation in the gene of the VHL ubiquitin ligase leads to an accumulation of the transcription factor hypoxia-induced factor (HIF) and tumor development.
- Mutations in the ubiquitin ligase Parkin have been identified in certain forms of Parkinson's disease .
- Mutations of Cullin7 E3 ubiquitin ligase were the cause of autosomal - recessive growth disorder 3-M syndrome identified.
literature
- Roland John Mayer, Aaron J. Ciechanover, Martin Rechsteiner: Protein Degradation (= The Ubiquitin-Proteasome System and Disease , Volume 4), Wiley-VCH, Weinheim 2008, ISBN 978-3-527-31436-2 .
- P. Ebner, GA Versteeg, F. Ikeda: Ubiquitin enzymes in the regulation of immune responses. In: Critical Reviews in Biochemistry and Molecular Biology. Volume 52, number 4, August 2017, pp. 425-460, doi : 10.1080 / 10409238.2017.1325829 , PMID 28524749 , PMC 5490640 (free full text) (review).
- A. Varshavsky: The Ubiquitin System, Autophagy, and Regulated Protein Degradation. In: Annual review of biochemistry. Volume 86, June 2017, pp. 123-128, doi : 10.1146 / annurev-biochem-061516-044859 , PMID 28654326 (review).
- PM Lombardi, MJ Matunis, C. Wolberger : RAP80, ubiquitin and SUMO in the DNA damage response. In: Journal of molecular medicine. Volume 95, number 8, August 2017, pp. 799-807, doi : 10.1007 / s00109-017-1561-1 , PMID 28681078 , PMC 5570449 (free full text) (review).
Individual evidence
- ↑ MJ Pearce, J. Mintseris et al. a .: Ubiquitin-like protein involved in the proteasome pathway of Mycobacterium tuberculosis. In: Science. Volume 322, number 5904, November 2008, pp. 1104-1107, doi : 10.1126 / science.1163885
- ↑ JA Maupin-Furlow: Prokaryotic ubiquitin-like protein modification. In: Annual review of microbiology. Volume 68, 2014, pp. 155-175, doi : 10.1146 / annurev-micro-091313-103447
- ^ G. Goldstein, M. Scheid, U. Hammerling, D. Schlesinger, H. Niall, E. Boyse: Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. In: Proc Natl Acad Sci USA . Volume 72, No. 1, January 1975, pp. 11-5; doi: 10.1073 / pnas.72.1.11 , PMID 1078892 , PMC 432229 (free full text).
- ↑ Information from the Nobel Foundation on the 2004 award ceremony to Avram Hershko and Irwin Rose (English)
- ↑ UniProt P62988
- ↑ Cecile M. Pickart, Shahri Raasi: Controlled Synthesis of Polyubiquitin Chains . In: Methods in Enzymology . Elsevier, 2005, ISBN 978-0-12-182804-2 , pp. 21-36 , doi : 10.1016 / s0076-6879 (05) 99002-2 ( elsevier.com [accessed May 27, 2018]).
- ^ A b C. M. Pickart, MJ Eddins: Ubiquitin: structures, functions, mechanisms. In: Biochim Biophys Acta . 1695 (1-3), November 29, 2004, pp. 55-72. PMID 15571809 .
- ↑ CM Pickart: Mechanisms underlying ubiquitination. In: Annu Rev Biochem . 70, 2001, pp. 503-533. PMID 11395416 .
- ↑ K. Cadwell, L. Coscoy: Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. In: Science. 309 (5731), July 1, 2005, pp. 127-130. PMID 15994556 .
- ^ A. Ciechanover, R. Ben-Saadon: N-terminal ubiquitination: more protein substrates join in. In: Trends Cell Biol . 14 (3), March 2004, pp. 103-106. PMID 15055197 .
- ↑ D. Mukhopadhyay, H. Riezman: Proteasome-independent functions of ubiquitin in endocytosis and signaling. In: Science. 315 (5809), Jan. 12, 2007, pp. 201-205. PMID 17218518 .
- ↑ A. Hershko, A. Ciechanover: The ubiquitin system for protein degradation. In: Annu Rev Biochem. 61, 1992, pp. 761-807. PMID 1323239 .
- ↑ H. Barriere, C. Nemes, K. Du, GL Lukacs: Plasticity of polyubiquitin recognition as lysosomal targeting signals by the endosomal sorting machinery. In: Mol Biol Cell. 18 (10), October 2007, pp. 3952-3965. PMID 17686993 .
- ↑ CM Pickart, D. Fushman: Polyubiquitin chains: polymeric protein signals. In: Curr Opin Chem Biol. 8 (6), December 2004, pp. 610-616. PMID 15556404 .
- ↑ S. Polo, S. Sigismund, M. Faretta, M. Guidi, MR Capua, G. Bossi, H. Chen, P. De Camilli, PP Di Fiore: A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. In: Nature . 416 (6879), March 28, 2002, pp. 451-455. PMID 11919637 .
- ↑ K. Flick, I. Ouni, YES Wohlschlegel, C. Capati, WH McDonald, JR Yates, P. Kaiser: proteolysis-independent regulation of the transcription factor MET4 by a single Lys 48-linked ubiquitin chain. In: Nat Cell Biol . 6 (7), July 2004, pp. 634-641. 20th June. PMID 15208638 .
- ^ A b C. Esser, S. Alberti, J. Höhfeld: Cooperation of molecular chaperones with the ubiquitin / proteasome system. In: Biochim Biophys Acta. 1695 (1-3), November 29, 2004, pp. 171-188. PMID 15571814 .
- ↑ RR Kopito: Biosynthesis and degradation of CFTR. In: Physiol Rev . 79 (1 Suppl), January 1999, pp. 167-173. PMID 9922380 .
- ↑ CL Ward, S. Omura, RR Kopito: Degradation of CFTR by the ubiquitin-proteasome pathway. In: Cell . 83 (1), October 6, 1995, pp. 121-127. PMID 7553863 .
- ↑ K. Robzyk, J. Recht, MA Osley: Rad6-dependent ubiquitination of histone H2B in yeast. In: Science. 287 (5452), Jan. 21, 2000, pp. 501-504. PMID 10642555 .
- ^ ZW Sun, CD Allis : Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. In: Nature. 418 (6893), Jul 4, 2002, pp. 104-108. PMID 12077605 .
- ↑ IL Goldknopf, H. Busch: Isopeptide linkage between nonhistone and histone 2 A polypeptides of chromosomal conjugate-protein A24. In: Proc Natl Acad Sci USA . 74 (3), March 1977, pp. 864-868. PMID 265581 .
- ^ LT Hunt, MO Dayhoff : Amino-terminal sequence identity of ubiquitin and the nonhistone component of nuclear protein A24. In: Biochem Biophys Res Commun . 74 (2), Jan. 24, 1977, pp. 650-655. PMID 836318 .
- ↑ SY Huang, MB Barnard, M. Xu, S. Matsui, SM Rose, WT Garrard: The active immunoglobulin kappa chain gene is packaged by non-ubiquitin-conjugated nucleosomes. In: Proc Natl Acad Sci U.S.A. 83 (11), June 1986, pp. 3738-3742. PMID 3012532 .
- ↑ M. Karin, Y. Ben-Neriah: Phosphorylation meets ubiquitination: the control of NF- [kappa] B activity. In: Annu Rev Immunol . 18, 2000, pp. 621-663. PMID 10837071 .
- ^ E. Meylan, J. Tschopp: The RIP kinases: crucial integrators of cellular stress. In: Trends Biochem Sci . 30 (3), March 2005, pp. 151-159. PMID 15752987 .
- ↑ TD Gilmore: Introduction to NF-kappaB: players, pathways, perspectives. In: Oncogene . 25 (51), October 30, 2006, pp. 6680-6884. PMID 17072321 .
- ↑ M. Magnani, R. Crinelli, M. Bianchi, A. Antonelli: The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB). In: Curr Drug Targets . 1 (4), December 2000, pp. 387-399. PMID 11467077 .
- ^ F. Bassermann, C. von Klitzing, S. Münch, RY Bai, H. Kawaguchi, SW Morris, C. Peschel, J. Duyster: NIPA defines an SCF-type mammalian E3 ligase that regulates mitotic entry. In: Cell. 122 (1), July 15, 2005, pp. 45-57. PMID 16009132 .
- ^ Y. Dang, LM Siew, YH Zheng: APOBEC3G is degraded by the proteasomal pathway in a Vif-dependent manner without being polyubiquitylated. In: J Biol Chem . March 6, 2008. PMID 18326044 .
- ↑ O. Karpiuk, Z. Najafova, F. Kramer, M. Hennion, C. Galonsk, A. König, N. Snaidero, T. Vogel, TA Shchebet, Y. Begus-Nahrmann, M. Kassem, M. Simons, H. Shcherbata, T. Beissbarth, SA Johnsen: The histone H2B monoubiquitination regulatory pathway is required for differentiation of multipotent stem cells. In: Molecular Cell. 46 (5), 2002, pp. 705-713.
- ↑ E. Weeber, J. Levenson, J. Sweatt: Molecular genetics of human cognition . In: Mol Interv . tape 2 , no. 6 , 2002, pp. 376-391, 339 , doi : 10.1124 / mi.2.6.376 , PMID 14993414 .
- ↑ H. Shimura, N. Hattori, S. Kubo, Y. Mizuno, S. Asakawa, S. Minoshima, N. Shimizu, K. Iwai, T. Chiba, K. Tanaka, T. Suzuki: Familial Parkinson disease gene product , parkin, is a ubiquitin protein ligase. In: Nat Genet . 25 (3), July 2000, pp. 302-305. PMID 10888878 .
- ↑ C. Huber, D. Dias-Santagata, A. Glaser, J. O'Sullivan, R. Brauner, K. Wu, X. Xu, K. Pearce, R. Wang, ML Uzielli, N. Dagoneau, W. Chemaitilly, A. Superti-Furga, H. Dos Santos, A. Mégarbané, G. Morin, G. Gillessen-Kaesbach, R. Hennekam, I. Van der Burgt, GC Black, PE Clayton, A. Read, M. Le Merrer, PJ Scambler, A. Munnich, ZQ Pan, R. Winter, V. Cormier-Daire: Identification of mutations in CUL7 in 3-M syndrome. In: Nat Genet. 37 (10), October 2005, pp. 1119-1124. PMID 16142236 .
Web links
- The Ubiquitin System for Protein Modification and Degradation. Information on Ubiquitin at Nottingham University.
- Jennifer McDowall: Ubiquitin. (English).