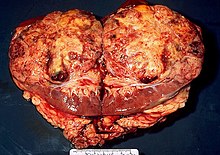
Kidney cancer
| Classification according to ICD-10 | |
|---|---|
| C64 | Malignant neoplasm of the kidney, excluding the renal pelvis |
| C65 | Malignant neoplasm of the renal pelvis |
| ICD-10 online (WHO version 2019) | |
Kidney cancer is the name given to a malignant kidney tumor. With 90% of adults, renal cell carcinoma (synonyms: renal carcinoma , adenocarcinoma of the kidneys , outdated: hypernephroma , hypernephroid carcinoma , Grawitz tumor ) originates from the proximal tubular cells ( epithelial cells ) (and not, as Grawitz wrongly assumed, from the adrenal gland). Nephroblastomas , lymphomas, and sarcomas of the kidneys are more common in childhood .
Kidney cancer is a relatively rare tumor disease (1 to 2% of all malignant tumors), in Germany around 9,500 men and 5,500 women are newly diagnosed each year (as of 2014). The kidney tumors are divided into benign ( benign ) and malignant ( malignant ). Malignant kidney tumors predominantly occur in the sixth and seventh decades of life .
Risk factors
Known risk factors for the occurrence of renal cell carcinoma are mainly smoking (also passive smoking ), obesity and high blood pressure ; Furthermore, chronic kidney failure , long-term analgesic therapy and congenital kidney diseases such as tuberous sclerosis or Hippel-Lindau's disease .
The exposure compared to trichlorethylene can cause renal cell carcinoma. If there was exposure to high doses for several years in the workplace, the resulting kidney cancer is considered an occupational disease (BK No. 1302: diseases caused by halogenated hydrocarbons). Similarly, cadmium and cadmium compounds and halogenated alkyl, aryl and Alkylaryloide as occupational carcinogens be responsible, this can also be recognized as an occupational disease.
classification
Under kidney cancer in the strict sense are malignant diseases that the functional tissue of the kidney (understands renal spring). In principle, one can at all portions nephron a malignant degeneration caused.
These types of kidney tumors are also known as renal cell carcinomas (NCC). A distinction is made according to the starting tissue, the cytogenetic findings and the histological picture. The most common is conventional renal cell carcinoma, which is often also referred to as clear cell carcinoma. Furthermore, the chromophilic (papillary), the chromophobic and, least of all, the Bellini duct carcinoma ( collecting tube carcinoma ) are found. The latter is characterized by a particular aggressiveness . The growth pattern of these tumors is also mentioned. Chromosomal aberrations (chromosome deviations) are described in terms of tumor cytogenesis . The cells of conventional renal cell carcinoma, for example, show a loss of fragments on chromosome 3 early in the development process .
Under renal cancer in a broader sense renal metastases are, for example, by the addition colon carcinoma or the lung understood.
Classification of epithelial neoplasms in the kidney
|
Type of carcinoma |
Growth pattern |
origin |
Cytogenetics |
|---|---|---|---|
|
Clear cell carcinoma |
acinous, sarcomatoid |
proximal tubule |
3p- |
|
papillary carcinoma |
papillary, tubular |
proximal tubule |
+7, +17, -Y |
|
chromophobic carcinoma |
solid, tubular, sarcomatoid |
Collecting tubule (cortical) |
|
|
oncocytic carcinoma |
Tumor nests |
Collecting tubule (cortical) |
- |
|
Bellini duct carcinoma |
papillary, sarcomatoid |
Collecting tubule (medullary) |
- |
In a broader sense, kidney cancer also includes malignant diseases that arise in the kidney, but do not arise from the functional tissue of the kidney. The renal pelvic carcinoma ( urothelial carcinoma of the renal pelvis) should be emphasized . This is a tumor that arises from the transitional tissue ( urothelium ) in the adjacent urinary tract.
The nephroblastoma ( Wilms tumor ) is also of particular importance . This is a mixed embryonic tumor that plays an important role in pediatrics ( pediatric oncology ). A possible preliminary stage is nephroblastomatosis .
Further malignant processes in the kidney can be caused by metastases ( lung cancer , breast cancer , malignant melanoma ) and occasionally by sarcomas .
Symptoms
The classic triad of blood in the urine ( hematuria ), flank pain and palpable tumor in the painful flank are rarely found. If the tumor invades the left renal vein, a symptomatic varicocele may form in the left testicle in men (1% of cases). Furthermore, paraneoplastic syndromes rarely occur (due to hormones formed in the tumor cells , such as renin , erythropoietin , parathyroid hormone or ACTH ). As with most cancers, general symptoms such as fatigue , fever, and weight loss can occur.
70% of kidney tumors are found by chance during imaging examinations ( sonography , computed tomography , etc.). In the last two decades, this has also led to a so-called “stage shift”: More and more often, small, not yet symptomatic tumors are found in the kidneys, which can therefore be treated better.
diagnosis
The clinical examination only shows large, advanced tumors in the abdomen. The laboratory testing may one through the blood loss through the urine caused anemia show. Sonography is the first step towards a more detailed assessment of the kidney. They can also be used to puncture suspicious masses in the kidney, which are then assessed histologically by the pathologist . The iv - urography is an x-ray with a kidney common contrast agents , which outcrops over a disabled urine flow can give and the function of the healthy kidney can be assessed. Computed tomography of the abdomen is performed to determine the spread of the tumor ( staging ) and thus its operability. With chest x-rays (chest x-ray ) and possibly with a skeletal scintigraphy and with a brain MRI ( magnetic resonance tomography ), possible distant metastases can be detected.
It is worth mentioning that radiographically, metastases from 1 cm in diameter can be recorded, which is clearly the preferred option for computed tomography.
| T | Tx | Primary tumor cannot be assessed | |
| T0 | No evidence of a primary tumor | ||
| T1 | Tumor limited to the kidney and ≤ 7 cm in its greatest extent | ||
| T1a | Tumor 4 cm or less in greatest extent | ||
| T1b | Tumor more than 4 cm, but no more than 7 cm in greatest extent | ||
| T2 | Tumor limited to the kidney and> 7 cm in the greatest extent | ||
| T2a | Tumor limited to the kidney and more than 7 cm, but no more than 10 cm in greatest extent | ||
| T2b | Tumor limited to the kidney, but> 10 cm in greatest extent | ||
| T3 | Tumor invades the surrounding tissue and the larger veins , but not the ipsilateral adrenal gland ( adrenal gland the same page), and not through the Gerota fascia also going | ||
| T3a | Tumor infiltrates the adrenal gland, its larger branches or the perirenal tissue, but does not penetrate beyond the Gerota fascia | ||
| T3b | Tumor infiltrates the renal vein (s) or the inferior vena cava to below the diaphragm | ||
| T3c | Tumor infiltrates the inferior vena cava above the diaphragm or invades the vein wall | ||
| T4 | Tumor infiltrates beyond the Gerota fascia and / or enters the adrenal gland on the same side | ||
| N | Nx | There can be no statement regionären lymph node metastases are taken | |
| N0 | No metastases in the regional lymph nodes | ||
| N1 | Metastases in the regional lymph nodes | ||
| M. | M0 | no distant metastases | |
| M1 | Distant metastases | ||
| Stage I. | T1 | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage III | T3 | N0 | M0 |
| T1, T2, T3 | N1 | M0 | |
| Stage IV | T4 | any N | M0 |
| any T | any N | M1 |
therapy
Surgical therapy
The treatment of choice in the presence of non-metastatic renal cell carcinoma is surgical removal of the tumor, although recent studies have shown that the long-term oncological results with kidney-preserving removal of the kidney tumor (if surgically possible and useful) are just as good as radical removal of the kidney.
For smaller tumors (stage T1a): In recent years, in addition to surgical therapy, minimally invasive forms of therapy have been used more and more often, which in T1a tumors (up to a maximum of 4 cm in diameter) have equally good results in treatment and in survival rate as well as in the showed disease-specific mortality. In these treatments, heating the tumor tissue (over 100 degrees) or freezing it leads to denaturation of the proteins and consequently to the destruction of the tumor cells, while at the same time the remaining healthy kidney tissue is spared. For this purpose, a small probe (needle) is inserted through the skin and into the tumor under image control (CT, US, etc.) and the tumor is treated with pinpoint accuracy. The people treated had a shorter hospital stay and fewer side effects. According to studies, the complication rate was lower compared to a surgical method (bleeding, infection, etc.). This method of "ablation" (RFA radio frequency ablation, microwave, cryotherapy, etc.), which are summarized as "thermal ablation", are further evaluated in studies and are currently recommended for inoperable, elderly patients. Promising studies will show whether this method can also be used in younger people.
With larger tumors ( stage II to IV) , the entire kidney with the adrenal gland , with the ureter , with the surrounding fatty tissue and with the capsule is surgically removed. Tumor cones that have grown into the renal vein ( vena renalis ) and the inferior vena cava must also be resected , if necessary with the use of a heart-lung machine as the tumor grows into the right atrium . Due to the latest findings, other standards are also in the current discussion: possibilities of laparoscopic radical nephrectomy or kidney-conserving partial resection. Adrenal removal is not always necessary. A kidney-preserving surgery is also useful if the other kidneys are healthy. There are minimally invasive therapy alternatives (RITA - Radiofrequency interstitial tumor ablation, HIFU - High-intensity focused ultrasound, etc.).
Also in metastatic renal cell carcinoma was verified by nephrectomy in combination with interferon 2b -α-better results are achieved than with interferon-α-2b alone. Nephrectomy is therefore also often performed in metastatic renal cell carcinoma. The CARMENA study compared sunitinib alone with sunitinib after nephrectomy. There was no deterioration without a nephrectomy. Removal of the kidney in the treatment of metastatic renal cell carcinoma with tyrosine kinase inhibitors is probably no longer an advantage.
Medical therapy
| drug | Substance class | operation area |
|---|---|---|
| Sunitinib | Tyrosine Kinase Inhibitor (TKI) |
First-line therapy at low risk |
| Pazopanib | TKI | First-line therapy at low risk |
| Cabozantinib | TKI | First-line therapy at high risk |
| Ipilimumab + nivolumab # |
monoclonal antibody |
First-line therapy at high risk |
| Bevacizumab | monoclonal antibody |
First-line therapy at low risk |
| Nivolumab |
monoclonal antibody |
Second line therapy |
| Axitinib | TKI | Second-line therapy at low risk |
| Sorafenib | TKI | Second-line therapy at low risk |
| Temsirolimus | mTOR inhibitor | First line therapy
only in rare cases |
| Everolimus | mTOR inhibitor | Second line therapy
only in rare cases |
| Interleukin-2 | Cytokine | First and second line therapy, only in very selected situations |
| Interferon alpha | Cytokine | Today, first and second line therapy is usually only used afterwards |
Drug therapies are used for locally inoperable or metastatic renal cell carcinoma. Therapy is then palliative ; healing can only be achieved in very rare exceptional cases. So far, no advantage of adjuvant drug therapy (i.e. supportive, after surgery) has been proven. In selected individual cases it may make sense to carry out a neoadjuvant drug therapy in order to achieve operability. However, this is not the standard therapy.
There has been significant change in recent years and a number of novel substances have been approved for treatment. Many of them have in randomized controlled trials , although a statistically for European approval generally significant prolongation of progression-free survival shown only in a few cases this has but also translates into a statistically significant improvement in overall survival (which is also partly in the study design was justified). A curative potential, for example in the form of long-lasting complete remissions of metastases, as reported in a few suitable patients under high-dose immunotherapy , has not yet been confirmed for these new active ingredients. Further substances from the group of tyrosine kinase inhibitors are currently in clinical testing ( cediranib ).
Conventional cytostatics
The classic substance classes of cytostatics ( anthracyclines , antimetabolites , alkylating agents , mitosis inhibitors , nucleoside analogs ) are largely ineffective in renal cell carcinoma. Renal cell carcinoma is therefore considered a chemotherapy-resistant carcinoma . The individual substances with the greatest effectiveness are vinblastine and 5-fluorouracil , each with about 7% response.
Immunotherapy
A cancer immunotherapy can be either non-specific (by stimulation of the immune system in the hope of eliciting an anti-tumor activity), or specifically, d. H. for example by tumor vaccines . With regard to non-specific immunotherapies, there have been attempts at therapy with interferon alpha (IFNα) and / or interleukin-2 (IL-2) since the 1980s . IFNα leads to response rates of 8 to 29% and an overall survival that is approximately 5 months longer than in untreated patients. Today, IFNα is considered the drug of choice because newer substances are more effective. The results of therapy with IL-2 are inconsistent, although this therapy has considerable side effects.
A review published in autumn 2007 describes the adjuvant administration of an autologous tumor vaccine. The recovered from the body's own tumor cells of the patient vaccine Reniale has in Phase III studies the period of progression-free survival improved and overall survival in renal cancer patients.
Clinical studies with the monoclonal antibodies against PD-1 ( nivolumab ) and CTLA-4 ( ipilimumab ) have shown very good results, especially in untreated high-risk patients. These so-called immune checkpoint inhibitors led to a higher response and to a longer overall survival in comparison with mTOR inhibitors or with tyrosine kinase inhibitors.
Oral tyrosine kinase inhibitors, bevacizumab
The in-depth molecular knowledge about the development of renal cell carcinoma has made it clear that certain so-called signal transduction pathways play an important role in tumor cells. These include signaling pathways in which the tyrosine kinases VEGFR , PDFGRA / B , FGFR1 etc. play a role. Therapy studies have shown that corresponding, more or less specific tyrosine kinase inhibitors can slow down tumor growth and in some cases also extend survival time. In Germany, two substances from this drug class are currently (as of December 2014) approved for renal cell carcinoma with a low or intermediate risk according to the MSKCC classification: sunitinib and pazopanib . The effectiveness of the two substances appears to be comparable and the median survival of both drugs in a large comparative study was around 28 months. The tyrosine kinase inhibitors sorafenib , axitinib and pazopanib (of course only if this was not given beforehand) are approved for second line therapy ( i.e. after the failure of a previous drug therapy) . Another drug that targets the tyrosine kinase signaling pathway is bevacizumab , a monoclonal antibody that targets the cytokine VEGF . Bevacizumab is also approved for first-line therapy in low-risk renal cell carcinoma (as of December 2014). All three drugs lead to a delay in the progression of the disease, sometimes by several months, but less than a year.
mTOR inhibitors
Everolimus is a so-called mTOR ( “mammalian target of rapamycin” ) inhibitor and has been shown to be effective in patients with high-risk renal cell carcinoma (according to the MSKCC classification). The drug also has first-line approval for this. Here, too, the delay in disease progression (“progression-free survival”) was a few months.
forecast
The (relative) 5-year survival rate of renal cell carcinoma is a good 77% overall, the (relative) 10-year survival rate a good 70/71%.
Depending on the stage:
- locally limited (T1-T2, N0, M0): 70–80%
- locally advanced (T3, N0-N2, M0): 20–60%
- Distant metastases (all T, all N, M1): <10%
To acute kidney failure or a chronic kidney failure does not occur as long as the kidney not affected any kidney disease has. As with living kidney donations, however, a tumor nephrectomy always leads to renal insufficiency of varying degrees. The glomerular filtration rate will first halve and then slowly rise to perhaps 60 or 70 percent of the preoperative baseline.
literature
- S3 guideline for renal cell carcinoma, diagnostics, therapy and follow-up care of the German Society for Urology (DGU) and the German Society for Hematology and Medical Oncology (DGHO). In: AWMF online (as of 2015)
Web links
- Renal Cell Cancer : Malignant Diseases of the Kidney , Cancer Information Service of the German Cancer Research Center (DKFZ), Heidelberg. January 29, 2013. Last accessed September 4, 2014.
- www.urologielehrbuch.de - Detailed chapter on renal cell carcinoma from the online urology textbook for doctors and medical professionals
- Kidney cancer , detailed patient information from a private self-help group "das Lebenshaus eV"
- J. Patrick Mershon et al .: Thermal ablation of the small renal mass: a critical analysis of current literature . In: Minerva Urologica E Nefrologica = The Italian Journal of Urology and Nephrology . December 12, 2019, doi : 10.23736 / S0393-2249.19.03572-0 , PMID 31833721 .
- Kidney cancer: basic information for patients. In: German Cancer Society . Retrieved January 15, 2020 .
- Modern immunotherapy for urological tumors. In: Cancer Information Service . July 18, 2019, accessed January 15, 2020 .
Individual evidence
- ↑ Maxim Zetkin , Herbert Schaldach: Lexicon of Medicine. 16th edition. Ullstein Medical, Wiesbaden 1999, ISBN 3-86126-126-X , p. 1409.
- ↑ a b c Cancer - Cancer in Germany 2013/2014 - frequencies and trends. (PDF) joint publication by the Robert Koch Institute and the Society of Epidemiological Cancer Registers in Germany V. , pp. 100-103 , accessed on October 10, 2018 .
- ↑ Willibald Pschyrembel: Clinical Dictionary. 267th edition, de Gruyter , Berlin / Boston 2017, ISBN 978-3-11-049497-6 , p. 1270.
- ↑ Jay D. Hunt et al .: Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies . In: International Journal of Cancer . tape 114 , no. 1 , March 10, 2005, ISSN 0020-7136 , p. 101-108 , doi : 10.1002 / ijc.20618 , PMID 15523697 .
- ↑ Entry on trichlorethylene in the GESTIS substance database of the IFA , accessed on July 19, 2012(JavaScript required) .
- ↑ Gerhard Triebig: renal cell carcinoma as an occupational disease. In: Deutsches Ärzteblatt . Volume 114, Issue 9, March 3, 2017, p. 160, doi: 10.3238 / arztebl.2017.0160a .
- ↑ B. Ljungberg et al .: EAU Guidelines on Renal Cell Carcinoma . In: European Association of Urology (EAU) . 2017, ISBN 978-90-79754-91-5 ( uroweb.org [PDF]).
- ↑ B. Ljungberg et al .: EAU Guidelines on Renal Cell Carcinoma . In: European Association of Urology (Ed.): Uroweb . 2018 ( uroweb.org [accessed October 10, 2018]).
- ↑ Frank Becker et al .: Important aspects of organ-preserving kidney tumor surgery: indications, new standard and oncological results . In: Deutsches Ärzteblatt Int . tape 106 , no. 8 , 2009, p. 117-122 ( aerzteblatt.de ). (PDF)
- ↑ Harry R. Marshall, Sepideh Shakeri, Melina Hosseiny, Anthony Sisk, James Sayre: Long-Term Survival after Percutaneous Radio Frequency Ablation of Pathologically Proven Renal Cell Carcinoma in 100 Patients . In: Journal of Vascular and Interventional Radiology . tape 31 , no. 1 , January 1, 2020, ISSN 1051-0443 , p. 15–24 , doi : 10.1016 / j.jvir.2019.09.011 , PMID 31767409 ( jvir.org [accessed January 15, 2020]).
- ↑ Xiaosong Meng, Rashed Ghandour, Vitaly Margulis: Tumor location does not limit percutaneous treatment of small renal masses with microwave ablation . In: Annals of Translational Medicine . tape 0 , no. 0 , August 27, 2019, ISSN 2305-5847 , p. 58 , doi : 10.21037 / 28515 ( amegroups.com [accessed January 15, 2020]).
- ↑ Devanshu Bansal, Rajeev Kumar: Percutaneous ablation for renal masses . In: Annals of Translational Medicine . tape 0 , no. 0 , May 8, 2019, ISSN 2305-5847 , p. 1 , doi : 10.21037 / 27794 ( amegroups.com [accessed January 15, 2020]).
- ↑ Marius Anglickis, Giedrė Anglickienė, Gintarė Andreikaitė, Arminas Skrebūnas: Microwave Thermal Ablation versus Open Partial Nephrectomy for the Treatment of Small Renal Tumors in Patients Over 70 Years Old . In: Medicina (Kaunas, Lithuania) . tape 55 , no. 10 , October 1, 2019, ISSN 1648-9144 , doi : 10.3390 / medicina55100664 , PMID 31581459 , PMC 6843191 (free full text).
- ^ Robert C. Flanigan et al .: Nephrectomy Followed by Interferon Alfa-2b Compared with Interferon Alfa-2b Alone for Metastatic Renal-Cell Cancer . In: The New England Journal of Medicine . tape 345 , no. 23 , December 6, 2001, ISSN 0028-4793 , p. 1655-1659 , doi : 10.1056 / NEJMoa003013 , PMID 11759643 .
- ^ A. Méjean et al .: Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma . In: The New England Journal of Medicine . tape 379 , no. 5 , 2018, p. 417-427 , doi : 10.1056 / NEJMoa1803675 .
- ^ Thomas Powles et al .: Updated European Association of Urology Guidelines: Recommendations for the Treatment of First-line Metastatic Clear Cell Renal Cancer . In: European Urology . tape 73 , no. 3 , March 2018, p. 311–315 , doi : 10.1016 / j.eururo.2017.11.016 ( elsevier.com [accessed April 20, 2018]).
- ↑ European Medicines Agency: Cabometyx (cabozantinib) Overview of Cabometyx and justification for approval in the EU. May 22, 2018, accessed June 10, 2019 .
- ↑ a b M. A. Reiter, M. Kurosch, A. Haferkamp: Renal cell carcinoma - drug therapy and prognostic models. In: Oncologist. 20, 2014, pp. 1241-1254. doi: 10.1007 / s00761-014-2784-1 .
- ↑ For example B. in many approval studies, a cross-over between the therapy arms is possible.
- ↑ DF McDermott: Immunotherapy of metastatic renal cell carcinoma. In: Cancer . 115, 2009, pp. 2298-2305.
- ↑ Chris Coppin et al .: Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomized trials . In: BJU international . tape 108 , no. 10 , November 2011, p. 1556–1563 , doi : 10.1111 / j.1464-410X.2011.10629.x , PMID 21952069 .
- ↑ P. Ivanyi et al .: New therapies for advanced renal cell carcinoma . In: Deutsches Ärzteblatt . No. 105 (13) , 2008, pp. 232-237 ( aerzteblatt.de ).
- ↑ Walter M. Stadler et al .: Prognostic factors for survival with gemcitabine plus 5-fluorouracil based regimens for metastatic renal cancer . In: The Journal of Urology . tape 170 , 4 Pt 1, October 2003, p. 1141–1145 , doi : 10.1097 / 01.ju.0000086829.74971.4a , PMID 14501711 .
- ↑ DF McDermott: Immunotherapy and targeted therapy combinations in renal cancer. In: Current clinical pharmacology. Volume 6, Number 3, August 2011, ISSN 2212-3938 , pp. 207-213. PMID 21827391 (Review).
- ↑ C. Doehn, AS Merseburger, D. Jocham, MA Kuczyk: Is there an indication for neoadjuvant or adjuvant system therapy for renal cell carcinoma? In: The Urologist. Volume 46, Number 10 / October 2007 doi: 10.1007 / s00120-007-1540-1 .
- ^ Matthias May et al .: Ten-year survival analysis for renal carcinoma patients treated with an autologous tumor lysate vaccine in an adjuvant setting . In: Cancer immunology, immunotherapy: CII . tape 59 , no. 5 , May 2010, p. 687-695 , doi : 10.1007 / s00262-009-0784-6 , PMID 19876628 .
- ^ Robert J. Motzer et al .: Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma . In: The New England Journal of Medicine . tape 378 , no. 14 , April 5, 2018, p. 1277–1290 , doi : 10.1056 / nejmoa1712126 ( nejm.org [accessed April 20, 2018]).
- ^ Robert J. Motzer et al .: Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma . In: The New England Journal of Medicine . tape 373 , no. 19 , November 4, 2015, p. 1803–1813 , doi : 10.1056 / nejmoa1510665 ( nejm.org [accessed April 20, 2018]).
- ^ Robert J. Motzer et al .: Pazopanib versus sunitinib in metastatic renal-cell carcinoma . In: The New England Journal of Medicine . tape 369 , no. 8 , 22 August 2013, p. 722-731 , doi : 10.1056 / NEJMoa1303989 , PMID 23964934 .