Nucleosides
Nucleosides (also nucleosides ) are organic molecules that consist of a nucleobase and a pentose . Different nucleosides occur in a cell , which differ in base or sugar content. In contrast to the nucleotides that make up nucleic acids (DNA or RNA), they do not contain any phosphate residues.
Basic types
The five basic types of nucleosides consist of either a purine or a pyrimidine base . If they are the building blocks of an RNA , the pentose (a simple sugar with five carbon atoms) is the ribose , in the DNA the pentose is the deoxyribose . That is why the building blocks of DNA are more precisely called deoxynucleosides, while nucleosides in the narrower sense are the building blocks of the various forms of RNA. The base and pentose are always linked with the purine bases via the nitrogen atom in position 9, with the pyrimidine bases via the nitrogen atom in position 1 and the C1 'atom of the sugar.
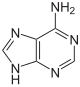
Purine bases
| Nucleobase | Nucleoside | Deoxynucleoside |
|---|---|---|
|
|
|
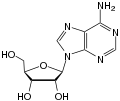
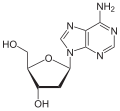
| Adenine | Adenosine , A. | Deoxyadenosine , dA |

|

|

|
| Guanine | Guanosine , G. | Deoxyguanosine , dG |
Note: Only one of the possible tautomeric structures is shown in each case .
Pyrimidine bases
| Nucleobase | Nucleoside | Deoxynucleoside |
|---|---|---|

|

|
|
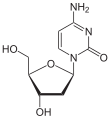
| Cytosine | Cytidine , C. | Deoxycytidine , dC |

|

|

|
| Thymine | Ribothymidine T (= 5-methyluridine) | Deoxythymidine , dT |

|

|

|
| Uracil | Uridine , U | Deoxyuridine , dU |
Modifications of the basic forms
In addition to these basic forms, there are also numerous modifications that can be found primarily in the tRNAs and rRNAs . Some are also found in DNA . These modified nucleosides usually only arise after transcription and are used to fine-tune the structure, activity and specificity of the molecules. Most modifications result from methylation .
The following selection is intended to provide an overview of the possible modifications. For comparison, the basic forms are also given:
Pyrimidine nucleosides
| Surname | symbol | Basic pyrimidine structure | R 1 | R 2 | R 3 | R 4 | R 5 | R 6 |
|---|---|---|---|---|---|---|---|---|
| Cytidine | C. |  |
Ribose | = O | -NH 2 | |||
| 3-methylcytidine | m 3 C | Ribose | = O | -CH 3 | -NH 2 | |||
| 5-methylcytidine | m 5 C | Ribose / deoxyribose | = O | -NH 2 | -CH 3 | |||
| N 4 -methylcytidine | m 4 C | Ribose / deoxyribose | = O | -NH-CH 3 | ||||
| N 4 , N 4 -dimethylcytidine | m 4 2 C | Ribose | = O | -N (CH 3 ) 2 | ||||
| 2'- O -methylcytidine | C m | 2'- O -methyl ribose | = O | -NH 2 | ||||
| Isocytidine (synth.) | iC | Ribose | -NH 2 | = O | ||||
| Pseudocytidine (synth.) | ΨC | -H | = O | -NH 2 | Ribose | |||
| Pseudoisocytidine (synth.) | psiC | -H | -NH 2 | = O | Ribose | |||
| 2-thiocytidine | s 2 C | Ribose | = S | -NH 2 | ||||
| N 4 -acetylcytidine | ac 4 C | Ribose | = O | -NH-CO-CH 3 | ||||
| Uridine | U | Ribose | = O | = O | ||||
| 3-methyluridine | m 3 U | Ribose | = O | -CH 3 | = O | |||
| 2'- O -methyluridine | U m | 2'- O -methyl ribose | = O | = O | ||||
| Pseudouridine | P, Ψ, Ψrd | -H | = O | = O | Ribose | |||
| Dihydrouridine | D, UH 2 , Uh | Ribose | = O | = O | -H, -H | -H, -H | ||
| 5-methoxyuridine | mo 5 U | Ribose | = O | = O | -O-CH 3 | |||
| 5- (carboxyhydroxymethyl) uridine | chm 5 U | Ribose | = O | = O | -CH (OH) -CO 2 CH 3 | |||
| 5-carboxymethylaminomethyl uridine | cmnm 5 U | Ribose | = O | = O | -CH 2 -NH-CH 2 -CO 2 CH 3 | |||
| 5-methylaminomethyl uridine | mnm 5 U | Ribose | = O | = O | -CH 2 -NH-CH 3 | |||
| 5-methoxycarbonylmethyl uridine | mcm 5 U | Ribose | = O | = O | -CH 2 -CO 2 CH 3 | |||
| 2-thiouridine | s 2 U | Ribose | = S | = O | ||||
| 4-thiouridine | s 4 U | Ribose | = O | = S | ||||
| Ribothymidine (= 5-methyluridine) | T, m 5 U | Ribose | = O | = O | -CH 3 | |||
| Dihydrothymidine | Deoxyribose | = O | = O | -H, -CH 3 | -H, -H |
Purine nucleosides
| Surname | symbol | Basic purine structure | R 1 | R 2 | R 3 | R 4 | R 5 | R 6 | R 7 | R 8 | R 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine | A. | 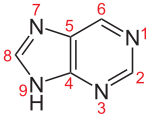 |
-NH 2 | Ribose | |||||||
| 1-methyladenosine | m 1 A | -CH 3 | -NH 2 | Ribose | |||||||
| 2-methyladenosine | m 2 A | -CH 3 | -NH 2 | Ribose | |||||||
| N 6 -methyladenosine | m 6 A | -NH-CH 3 | Ribose / deoxyribose | ||||||||
| N 6 , N 6 -dimethyladenosine | m 6 2 A | -N (CH 3 ) 2 | Ribose | ||||||||
| 2'- O -methyladenosine | A m | -NH 2 | 2'- O -methyl ribose | ||||||||
| Inosine | I. | = O | Ribose | ||||||||
| 1-methylinosine | m 1 I | -CH 3 | = O | Ribose | |||||||
| 2'- O -methylinosine | I m | = O | 2'- O -methyl ribose | ||||||||
| Guanosine | G | -NH 2 | = O | Ribose | |||||||
| 1-methylguanosine | m 1 G | -CH 3 | -NH 2 | = O | Ribose | ||||||
| 7 + -Methylguanosin | m 7 G | -NH 2 | = O | -CH 3 | Ribose | ||||||
| N 2 -methylguanosine | m 2 G | -NH-CH 3 | = O | Ribose | |||||||
| N 2 , N 2 -dimethylguanosine | m 2 2 G | -N (CH 3 ) 2 | = O | Ribose | |||||||
| 2'- O -methylguanosine | G m | -NH 2 | = O | 2'- O -methyl ribose | |||||||
| Isoguanosine (synth.) | iG | = O | -NH 2 | Ribose |
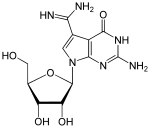
Hypermodified nucleosides and with a modified basic structure

|
|
|
Queuosine (Q, picture above) β- D - galactosyl -queuosine (galQ) β- D - mannosyl -queuosine (manQ) |
Archaeosine (G * , only occurs in archaea ) |

|

|
|
2′- O -ribosyladenosine phosphate (Ar (p), rAMP) only found in eukaryotes |
Wybutosin (Y, yW; picture above) Wyosin (Wyo, imG) |

|
|
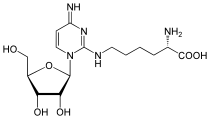
| N 6 -Threonylcarbamoyladenosine (t 6 A) | Lysidine (k 2 C) |
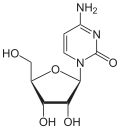
Variations of the pentoses
| Nucleobase | (Ribosyl) nucleoside | Deoxynucleoside | Arabinosyl nucleoside | (Methyl ribosyl) nucleoside |
|---|---|---|---|---|

|
|

|

|

|
| Cytosine | Cytidine , C. | Deoxycytidine , dC | Cytarabine , araC | 2'- O -methylcytidine , C m |
physiology
If the hydroxyl group of the C-5 atom of the pentose of a nucleoside is esterified with phosphate, the corresponding nucleotide is formed . Depending on the number of phosphate residues, one speaks of mono-, di- and triphosphates. The central importance of the nucleotides results in the same meaning for the corresponding nucleosides, since they can be converted into these as building blocks of the nucleotides.
The nucleosides are available in all living things by splitting off the last phosphate group in the nucleotides by means of hydrolysis , with the help of nucleotidase enzymes. Furthermore, inosine can be synthesized from adenosine using AMP deaminase or guanine deaminase . Xanthosine is accordingly obtainable not only by hydrolysis of XMP, but also from guanosine by means of guanosine deaminase .
The degradation takes place via nucleosidases to the nucleobase and in the case of purines via xanthine to uric acid or in the case of pyrimidines to alanine or 2-aminobutyric acid .
Nucleoside analogs
Nucleoside analogs play a major role in antiretroviral therapy (see AIDS ). A number of modern antivirals contain these substances. Best known is the active ingredient acyclovir , which is often used against herpes simplex viruses (HSV-1 and -2). Ganciclovir , which, like acyclovir, is a guanosine analogue and specifically suppresses the replication of CMV , is also widespread .
Other base analogs that are prescribed against viral infections are e.g. B. zidovudine (also azidothymidine, AZT for short), stavudine , zalcitabine , diadenosine , idoxuridine , fluridine and ribavirin .
Azidothymidine
Azidothymidine (AZT, INN : zidovudine ) was the first effective drug against the HI virus . Since it has an azido group instead of a hydroxyl group on the 3'-carbon of the ribose, the chain can no longer grow here during the synthesis of the virus DNA and an inactive provirus is created .
However, some of these drugs also show considerable side effects, which is why they are only conditionally suitable for long-term therapy.
Puromycin
Puromycin (also Purimycin) is a nucleoside antibiotic obtained from the bacterium Streptomyces alboniger , which inhibits protein synthesis and is effective against some tumors , amoebas , trypanosomes and worms. But since it is too toxic for humans, it is only used in microbiological experiments . Structurally, it is derived from adenosine.
Nucleoside analogs with arabinose
Nucleoside analogs with arabinose ( arabinosyl nucleosides ) are mostly used as cytostatics .
Individual evidence
- ↑ Löffler, Petrides, Heinrich: Biochemistry and Pathobiochemistry. 8th edition. Springer, Heidelberg 2007, ISBN 978-3-540-32680-9 .
- ↑ Patrick A. Limbach, Pamela F. Crain, James A. McCloskey: "Summary: the modified nucleosides of RNA", Nucleic Acids Research , 1994 , 22 (12), pp. 2183-2196 ( doi: 10.1093 / nar / 22.12 .2183 , PMC 523672 (free full text), PMID 7518580 ).
- ↑ Melanie Ehrlich, Miguel A. Gama-Sosa, Laura H. Carreira, Lars G. Ljungdahl, Kenneth C. Kuo, Charles W. Gehrke: "DNA methylation in thermophilic bacteria: N 4 -methylcytosine, 5-methylcytosine, and N 6 -methyladenine ", Nucleic Acids Research , 1985 , 13 (4), pp. 1399-1412 ( doi: 10.1093 / nar / 13.4.1399 , PMC 341080 (free full text), PMID 4000939 ).




