Stavudine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
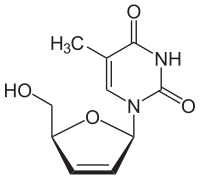
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Stavudine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 12 N 2 O 4 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| Mechanism of action | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 224.21 g · mol -1 | ||||||||||||||||||
| Melting point | |||||||||||||||||||
| solubility |
soluble in water (6.6 g l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Stavudine (d4T) (trade name: Zerit ® ; manufacturer: Bristol-Myers Squibb ) is a drug for the treatment of HIV- 1 infected patients as part of an antiretroviral combination therapy , but is rarely used in western industrialized countries today.
It belongs to the group of nucleoside reverse transcriptase inhibitors (NRTIs).
properties
Stavudine can come in four different crystal forms. The polymorphic forms I, II and IV differ in terms of their melting points at 168.1 ° C, 165.5 ° C and 169.5 ° C. Form I is the thermodynamically stable form at room temperature . Form III is 1/3 hydrate with a stoichiometric ratio of active ingredient to water of 3: 1 in the crystal lattice .
pharmacology
The antiretroviral activity of stavudine has been demonstrated in vitro . The substance is about 5–10 times weaker than zidovudine in vitro . In combination with other antiviral agents, synergistic effects could be demonstrated. The development of stavudine-resistant HI viruses has been demonstrated in vitro.
Pharmacokinetics
The bioavailability of stavudine is good. About 90% are absorbed orally. Simultaneous consumption with food has only minor, clinically irrelevant, effects. After ingestion of 40 mg, peak concentrations of approx. 0.8–1.0 mg / l are achieved in the plasma. CSF concentrations: up to approx. 70% of the plasma concentrations. Elimination half-life: approx. 1.6 hours. Intracellular half-life of the biologically active triphosphate: approx. 3.5 hours.
Side effects
Strongly mitochondrially toxic, lipoatrophy , peripheral neuropathies (PNP), especially in combination with DDI, rarely diarrhea , nausea, headache, hepatic steatosis , pancreatitis, very rarely: lactic acidosis . Contraindicated in PNP. Avoid neurotoxic drugs (ethambutol, cisplatin, INH, vincristine etc.).
development
The substance was synthesized in 1966 by Jerome P. Horwitz .
Web links
- AIDSMeds.com
- Information for doctors and pharmacists on rational infection therapy : stavudine , another nucleoside analogue for the treatment of HIV infection
Individual evidence
- ↑ a b c d Lu, J .; Rohani, S .: Polymorphic Crystallization and Transformation of the Anti-Viral / HIV Drug Stavudine in Org Process Res Dev . 13 (2009) 1262-1268, doi : 10.1021 / op900004c .
- ↑ a b Entry on stavudine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Data sheet 2 ′, 3′-Didehydro-3′-deoxythymidine, ≥98% (TLC) from Sigma-Aldrich , accessed on November 18, 2019 ( PDF ).
- ^ JR Horwitz, J. Chua, MA Da Rooge, M. Noel, IL Klundt: Nucleosides. IX. The formation of 2 ', 2'-unsaturated pyrimidine nucleosides via a novel beta-elimination reaction. In: The Journal of organic chemistry. Volume 31, Number 1, January 1966, pp. 205-211, PMID 5900814 .