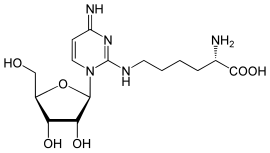
Lysidine (nucleoside)
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Surname | Lysidine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 15 H 25 N 5 O 6 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 371.39 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Lysidine (k 2 C) is a rare nucleoside and occurs in the tRNA . It consists of β- D- ribofuranose (sugar) and a derivative of cytosine , with the carbonyl group being replaced by the amino acid lysine . This substitution pattern is similar to that of the nucleoside agmatidine .
properties
Bacteria decode the isoleucine codon AUA with a tRNA that was changed from cytidine to lysidine at the third position of the anticodon (position 34). Cytidine usually pairs with guanosine , whereas lysidine only pairs with adenosine . Uridine was not incorporated at this point, although it is the usual partner for adenosine; however, it can also form a " wobble base pair " with guanosine. Because of its uniqueness in the base pairing , lysidine therefore ensures better translation reliability .
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Patrick A. Limbach, Pamela F. Crain, James A. McCloskey: "Summary: the modified nucleosides of RNA", Nucleic Acids Research , 1994 , 22 (12), pp. 2183-2196 ( doi : 10.1093 / nar / 22.12 .2183 , PMC 523672 (free full text), PMID 7518580 ).
- ↑ K. Nakanishi, S. Fukai, Y. Ikeuchi, A. Soma, Y. Sekine, T. Suzuki, O. Nureki: "Structural basis for lysidine formation by ATP pyrophosphatase accompanied by a lysine-specific loop and a tRNA recognition domain ”, Proc. Natl. Acad. Sci. USA , 2005 , 102 (21), pp. 7487-7492 ( doi : 10.1073 / pnas.0501003102 , PMC 1140429 (free full text), PMID 15894617 ).
- ↑ SP Salowe, J. Wiltsie, JC Hawkins, LM Sonatore: "The Catalytic Flexibility of tRNA Ile- lysidine Synthetase Can Generate Alternative tRNA Substrates for Isoleucyl-tRNA Synthetase", J. Biol. Chem. , 2009 , 284 (15), Pp. 9656-9662 ( doi : 10.1074 / jbc.M809013200 , PMC 2665086 (free full text), PMID 19233850 ).
Web links
- Modification Summary of Lysidine in the Modomics database, accessed January 14, 2014.
