Immune checkpoint inhibitor
An immune checkpoint inhibitor (also immune checkpoint inhibitor ) is a molecule having an immune checkpoint inhibits. In oncology , anti-inflammatory immune checkpoints are blocked. These molecules are used in immunotherapy and are currently only monoclonal antibodies .
properties
The immune system has both costimulatory (activating) and inhibitory (inhibitory) signaling pathways. These regulatory mechanisms influence the strength and intensity of an immune response . Normally, these mechanisms serve u. a. the avoidance of autoimmune reactions . Those signaling pathways with an inhibitory effect are referred to as co-inhibitory immune checkpoints and cause a downregulation of the T cell activation or the T cell effector function. The immune checkpoints with an inflammatory effect are referred to as co-stimulatory immune checkpoints.
Treatment of malignancies with immune checkpoint inhibitors
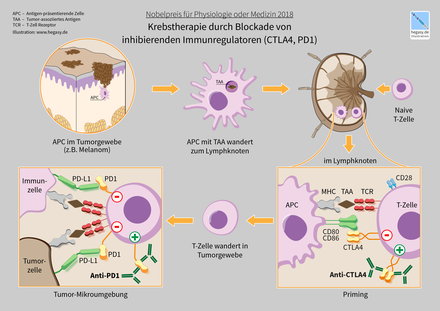
With regard to tumor diseases , it is known that tumor cells can use these immune checkpoints in order to escape recognition by the immune system ( immune evasion ). This immunosuppressive milieu of some tumors is generated by the production of inhibitory cytokines , the recruitment of immunosuppressive immune cells and the upregulation of co-inhibitory receptors , the negatively regulating immune checkpoints . This knowledge led to the development of checkpoint inhibitors which “switch off” immunosuppressive signals by breaking a receptor-ligand bond. In this way, the immune system of the organism can recognize and fight the degenerate cancer cells.
Treatment with checkpoint inhibitors has revolutionized oncology in recent years. Metastatic tumors such as melanoma , the course of which has so far been little influenced, have become treatable. For other tumors, such as NSCLC (non-small cell lung cancer), treatment with checkpoint inhibitors could replace chemotherapy with many side effects, and in some cases even surpass it. However, checkpoint inhibitors can also have significant side effects. The partial deactivation of the "friend-foe recognition" of killer cells can lead to life-threatening autoaggressive diseases, e.g. B. the lungs, the liver or the kidneys. Numerous studies on treatment with checkpoint inhibitors have been ongoing since the beginning of the 2010 years . Therefore, the evidence of effectiveness and the approval status of the preparations can change at short notice. The following drugs are used or tested:
| effect | Pharmacon | Trade name | Manufacturer | Malignancies |
|---|---|---|---|---|
| CTLA-4 inhibitor | Ipilimumab | Yervoy | Bristol-Myers Squibb | Melanoma, renal cell carcinoma |
| Tremelimumab | not yet approved | Medimmune ( Astra Zeneca ) | Testing with various entities | |
| PD-1 antibody | Nivolumab | Opdivo | Bristol-Myers Squibb | malignant melanoma, NSCLC , renal cell carcinoma , Hodgkin's lymphoma , hepatocellular carcinoma , ENT squamous cell carcinoma , urothelial carcinoma , colorectal carcinoma |
| Pembrolizumab | Keytruda | Merck Sharp & Dohme | malignant melanoma, NSCLC, renal cell carcinoma, Hodgkin's lymphoma, ENT squamous cell carcinoma, urothelial carcinoma | |
| Cemiplimab | Libtayo | Sanofi / Regeneron | Metastatic or locally advanced squamous cell carcinoma of the skin | |
| Spartalizumab | not yet approved | Novartis | Metastatic melanoma | |
| PD-L1 antibody | Atezolizumab | Tecentriq | Roche | NSCLC, renal cell carcinoma, urothelial carcinoma, breast carcinoma |
| Durvalumab | Imfinzi | Astra Zeneca | Urothelial carcinoma, NSCLC | |
| Avelumab | Bavencio | Merck KGaA | Urothelial carcinoma, Merkel cell carcinoma |
The tumors listed in the table were successfully tested. However, this does not mean that they are effective in all cases and that they are approved. The latter can be found in the articles for the individual preparations.
Immune checkpoint inhibitors are z. B. Antibodies against CTLA-4 (ipilimumab), PD-1 (nivolumab) and PD-L1 (atezolizumab, durvalumab and avelumab). After the antibody has been infused, the antibody is bound to these proteins, which act as immune checkpoints. As a result, the cells that carry one of these proteins on the cell surface and bind the antibody are temporarily (or as long as the therapeutic antibody is circulating in the body) attacked by immune cells and removed from the body by macrophages (temporary cell depletion ). These processes lead to an intensification of the immune response against the tumor, so that its strategy of immune evasion is counteracted. Therefore, immune checkpoint inhibitors are used in cancer immunotherapy , including in combination with cancer vaccines .
The PD-L1 antibody avelumab was compared with the VEGF inhibitor axitinib in advanced renal cell carcinoma in the JAVELIN Renal 101 study , a randomized phase III study, with the tyrosine kinase inhibitor sunitinib . A significantly longer recurrence-free survival was achieved with avelumab and axitinib. In patients with PD-L1 positive tumors, the median recurrence-free survival was 13.8 months with avelumab and axitinib and 7.2 months with sunitinib. Even when including the PL-L1-negative tumor cases, equally good results were achieved.
Therapeutic monoclonal antibodies (initially the anti-CTLA-4 antibody ipilimumab and then the anti-PD-1 antibodies nivolumab and pembrolizumab) were developed against these immune-reaction-reducing molecules and initially evaluated in studies on metastatic malignant melanoma . Treatment with these antibodies showed a clinical effect that was not observed in comparison with the usual therapies and also a different side effect profile characterized by autoimmune phenomena .
Nobel Prize for the discovery of immune checkpoints
In 2018, James Patrick Allison and Tasuku Honjo received the Nobel Prize in Physiology or Medicine . Allison and his group at the University of California at Berkeley discovered CTLA-4 and found that this protein weakened the immune response. Allison was able to use a mouse model to show that this mechanism can be used to fight cancer. Tasuku Honjo and his research group discovered that PD-1 has a dampening effect on the immune response. Based on this research, a number of effective drugs for treating cancer have been developed. Honjo recognized that dampening the immune response could also be useful in the case of autoimmune diseases , allergies and transplant rejection .
Applications
Immune checkpoint inhibitors are currently used in the treatment of melanoma , non-small cell lung cancer, and clear cell renal cell carcinoma and squamous cell carcinoma.
literature
- Patrick Terheyden, Angela Krackhardt, Thomas Eigenler: System therapy of melanoma. Use of immune checkpoint inhibitors and inhibition of intracellular signal transduction. In: Deutsches Ärzteblatt. Volume 116, Issue 29 f., (July 22) 2019, pp. 497–504.
Individual evidence
- ↑ a b c S. Ceeraz, EC Nowak, CM Burns, RJ Noelle: Immune checkpoint receptors in regulating immune reactivity in rheumatic disease. In: Arthritis research & therapy. Volume 16, number 5, 2014, p. 469. PMID 25606596 , PMC 4289356 (free full text).
- ^ DocCheck Medical Services GmbH: Checkpoint Inhibitor. Retrieved June 2, 2019 .
- ^ Immune checkpoint inhibitors - Altmeyers Encyclopedia - Department of Dermatology. April 18, 2018, accessed June 2, 2019 .
- ↑ Cancer Information Service, German Cancer Research Center: PD-1 inhibitors: Immunotherapy against cancer. Retrieved June 2, 2019 .
- ↑ Edition 02/2017. Accessed June 2, 2019 .
- ↑ "A completely new approach in cancer therapy". Retrieved June 2, 2019 .
- ^ Avoxa media group Deutscher Apotheker GmbH: Checkpoint inhibitors: Immunotherapy against cancer. Retrieved June 2, 2019 .
- ↑ Checkpoint Inhibitors • ARZNEI-NEWS.de. Retrieved June 2, 2019 .
- ↑ Robert J. Motzer, Nizar M. Tannir, David F. McDermott, Osvaldo Arén Frontera, Bohuslav Melichar: Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma . In: New England Journal of Medicine . tape 378 , no. 14 , April 5, 2018, ISSN 0028-4793 , p. 1277–1290 , doi : 10.1056 / NEJMoa1712126 , PMID 29562145 , PMC 5972549 (free full text) - ( nejm.org [accessed May 11, 2019]).
- ↑ Pipeline - AstraZeneca. Retrieved August 15, 2019 .
- ^ Annette Mende: The third PD-1 inhibitor. In: Pharmaceutical newspaper. September 4, 2019, accessed November 8, 2019 .
- ↑ Dr. Frank Antwerpes: Spartalizumab. DocCheck Flexikon, accessed on November 8, 2019 .
- ↑ K. Shih, HT Arkenau, JR Infante: Clinical impact of checkpoint inhibitors as novel cancer therapies. In: Drugs. Volume 74, number 17, November 2014, pp. 1993-2013, doi: 10.1007 / s40265-014-0305-6 . PMID 25344022 , PMC 4224737 (free full text).
- ^ A. Ito, S. Kondo, K. Tada, S. Kitano: Clinical Development of Immune Checkpoint Inhibitors. In: BioMed research international. Volume 2015, p. 605478, doi: 10.1155 / 2015/605478 . PMID 26161407 , PMC 4486755 (free full text).
- ↑ MK Callahan, MA Postow, JD Wolchok: Targeting T Cell Co-receptors for Cancer Therapy. In: Immunity. Volume 44, Number 5, May 2016, pp. 1069-1078, doi: 10.1016 / j.immuni.2016.04.023 . PMID 27192570 .
- ^ KR Rajani, RG Vile: Harnessing the Power of Onco-Immunotherapy with Checkpoint Inhibitors. In: Viruses. Volume 7, number 11, 2015, pp. 5889-5901, doi: 10.3390 / v7112914 . PMID 26580645 .
- ↑ J. KLEPONIS, R. Skelton, L. Zheng: Fueling the engine and releasing the break: combinational therapy of cancer vaccines and immune checkpoint inhibitors. In: Cancer biology & medicine. Volume 12, number 3, September 2015, pp. 201-208, doi: 10.7497 / j.issn.2095-3941.2015.0046 . PMID 26487965 , PMC 4607816 (free full text).
- ↑ Robert J Motzer, Konstantin Penkov, John Haanen, Brian Rini, Laurence Albiges: Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma . In: New England Journal of Medicine . tape 380 , no. 12 , March 21, 2019, ISSN 0028-4793 , p. 1103–1115 , doi : 10.1056 / NEJMoa1816047 ( nejm.org [accessed March 26, 2019]).
- ^ I. Márquez-Rodas, P. Cerezuela, A. Soria, A. Berrocal, A. Riso, M. González-Cao, S. Martín-Algarra: Immune checkpoint inhibitors: therapeutic advances in melanoma. In: Annals of translational medicine. Volume 3, number 18, October 2015, p. 267, doi: 10.3978 / j.issn.2305-5839.2015.10.27 . PMID 26605313 , PMC 4630549 (free full text).
- ↑ BA Teply, EJ Lipson: Identification and management of toxicities from immune checkpoint-blocking drugs. In: Oncology. Volume 28 Suppl 3, November 2014, pp. 30-38. PMID 25384885 .
- ↑ J. Villadolid, A. Amin: Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. In: Translational lung cancer research. Volume 4, Number 5, October 2015, pp. 560-575. doi: 10.3978 / j.issn.2218-6751.2015.06.06 . PMID 26629425 . PMC 4630514 (free full text).
- ↑ AA Hurwitz, DR Leach, A van Elsas, SE Townsend, JP Allison: Manipulation of T cell activation in the anti-tumor immune response. In: E Mihich, C Croce (eds.): The Biology of Tumors. Plenum Press, New York, pp. 2013-2019 .
- ↑ Arthur A Hurwitz, Barbara A Foster, Eugene D Kwon, Tan Truong, Eugene M Choi, Norman M Greenberg, Maurice B Burg, James P Allison: Combination Immunotherapy of Primary Prostate Cancer in a Transgenic Mouse Model Using CTLA-4 Blockade1. In: CANCER RESEARCH . tape 60 , 2000, pp. 2444-2448 .
- ↑ Y Ishida, Y Agata, K Shibahara, T Honjo: Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. In: EMBO J . tape 11 , 1992, pp. 3887 .
- ^ T Okazaki, T Honjo: PD-1 and PD-1 ligands: from discovery to clinical application . In: International Immunology . tape 19 , no. 7 , June 22, 2007, ISSN 0953-8178 , p. 813-824 , doi : 10.1093 / intimm / dxm057 ( oup.com [accessed April 5, 2019]).
- ↑ FS Hodi et al.: Improved survival with ipilimumab in patients with metastatic melanoma. In: N Engl J Med. 363, 2010, pp. 711-723. doi: 10.1056 / NEJMoa1003466 . PMID 20525992 ; PMC 3549297 (free full text)
- ↑ J. Brahmer et al.: Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. In: N Engl J Med. 373, 2015, pp. 123-135. doi: 10.1056 / NEJMoa1504627 .
- ↑ H. Borghaei include: Nivolumab versus docetaxel in advanced nonsquamous Non-Small-Cell Lung Cancer. In: N Engl J Med. 373, 2015, pp. 1627-1639. doi: 10.1056 / NEJMoa1507643 .
- ↑ EB Garon et al: Pembrolizumab for the treatment of non-small-cell lung cancer. In: N Engl J Med. 372, 2015, pp. 2018-2028. doi: 10.1056 / NEJMoa1501824 .
- ↑ RJ Motzer, B. Escudier, DF McDermott, S. George, HJ Hammers, S. Srinivas, SS Tykodi, JA Sosman, G. Procopio, ER Plimack, D. Castellano, TK Choueiri, H. Gurney, F. Donskov, P. Bono, J. Wagstaff, TC Gauler, T. Ueda, Y. Tomita, FA Schutz, C. Kollmannsberger, J. Larkin, A. Ravaud, JS Simon, LA Xu, IM Waxman, P. Sharma: Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. In: The New England Journal of Medicine . Volume 373, number 19, November 2015, pp. 1803-1813, doi: 10.1056 / NEJMoa1510665 . PMID 26406148 .
- ↑ European Commission Approves Bristol-Myers Squibb's Opdivo (nivolumab) for Squamous Cell Cancer of the Head and Neck in Adults Progressing On or After Platinum-based Therapy PM BSM of April 28, 2017, accessed on May 11, 2017.