Multiple myeloma
| Classification according to ICD-10 | |
|---|---|
| C90.0 | Multiple myeloma |
| C90.3 | Solitary plasmacytoma |
| ICD-10 online (WHO version 2019) | |
| Classification according to ICD-O-3 | |
|---|---|
| 9732/3 | Multiple myeloma (C42.1) |
| 9731/3 | Plasmacytoma onA, solitary myeloma |
| ICD-O-3 first revision online | |
The Multiple Myeloma ( MM ), also briefly myeloma , often used synonymously myeloma (formerly myelogenes plasmacytoma ) and less common: plasma cell myeloma , Kahler's disease or Kahlersche disease after Otto Kahler and Huppert's disease by Karl Hugo Huppert , is one of the plasma cells of the bone marrow outgoing cancer , a non-Hodgkin's lymphoma of the hematopoietic system . The MM is ranked among the monoclonal gammopathies . It is characterized by a pathological (uncontrolled) increase in antibody- producing cells , the plasma cells (differentiated B cells ). The degenerate plasma cells usually produce antibodies or parts / fragments thereof (e.g. free light chains ). Since a malignant plasma cell clone is derived from a common precursor cell, they are genetically largely identical and also produce identical (= monoclonal ) antibodies. Closely related, but to be distinguished from multiple myeloma, are plasma cell leukemia, extramedullary plasmacytoma, and solitary plasmacytoma.
Multiple myeloma is always preceded by monoclonal gammopathy of unclear significance (MGUS). MGUS does not necessarily have to lead to malignant disease. Often, however, various comorbidities / complications are associated with this even without the development of a haematological disease. As the transition stage between MGUS and MM applies the smoldering multiple myeloma ( smoldering myeloma ; SMM). The degree of malignancy of MM can be very different and ranges from slowly progressing MM to an aggressive, rapidly progressing form, which is often characterized by dedifferentiated, plasmoblastic plasma cells.

On terminology and medical history
Classification in the system of haemato-oncological diseases
Multiple myeloma is one of the indolent (low-grade) B-cell non-Hodgkin lymphomas . The term plasmacytoma is to be distinguished from real multiple myeloma , which only describes an isolated myeloma in which the disease is limited to a single local focus of the disease (usually osteolysis = local bone dissolution):
“In the Anglo-American language area, the disease known as 'multiple myeloma' is strictly separated from the 'plasmocytoma'. This separation should also be made in the German terminology , because according to the definition a 'plasmacytoma' is a 'solitary tumor made of plasma cells' ... If there are several plasma cell tumors, the term 'multiple myeloma' best describes this form of plasma cell disease. "
The ICD-10 classification of the WHO strictly distinguishes between C90.0 multiple myeloma and C90.03 solitary plasmacytoma .
In Germany, however, a largely synonymous use of the two terms is common in general clinical usage, which is why plasmacytoma often means multiple myeloma.
The precursors of smoldering multiple myeloma (SMM) and monoclonal gammopathy of unclear significance (MGUS), which also have clonal immunoglobulin production, are to be distinguished from multiple myeloma / plasmacytoma. Symptoms and complications can also occur in the preliminary stages, but this does not necessarily lead to the diagnosis of multiple myeloma due to the lack of criteria . In order to enable a more differentiated assessment of the patients, the term monoclonal gammopathy of renal significance (MGRS) was introduced for patients with nephrological complications that can be traced back to clonal immunoglobulin production by pathologically altered plasma cells . Other monoclonal gammopathies that are not referred to as multiple myeloma are AL amyloidosis (but this can also occur in addition to multiple myeloma) and Waldenström's disease . For some time, a reform of the terminology has been discussed due to the complexity of the disease (s) and the sometimes difficult to distinguish preliminary stages as well as closely related entities. A distinction should be made here between monoclonal gammopathy, which requires treatment, and one that is only under observation without complications.
etymology
The medical term 'myel-om', translated 'bone marrow tumor', is a neologism , a word formation created by derivation from the noun ancient Greek μυελός “myelós”, 'bone marrow' and the suffix '-om', from ancient Greek ωμ (α) "ōm (a)".
However, the suffix “-om”, borrowed from Greek, is not a lexeme , but a word formation morpheme with no conceptual meaning. A lexeme, i.e. a noun , a noun “ωμ (α)”, “ōm (a)” never existed in ancient Greek.
In modern medical terminology, on the other hand, this suffix '-om' takes on the rank of a lexeme , an appellative that has internationally been assigned the meaning of tumor .
“At the time when modern medicine needed a more differentiated terminological reservoir as a correlate for the tumors, which were more and more specified by location, histological structure, etc. and was adapting Greek and / or Latin material for neologisms in a tried and tested manner, it was obvious to use the common To make the denominator in the matter (= tumor ) also formally visible as a constant element of word formation. So one chose the group of formations on "ωμ (α)", "ōm (a)" of ancient Greek ("καρκίν-ωμα", Karkinōma, "σάρκωμα", sarcoma, etc.) as a pattern and then formed the purely Greek terms carcinoma , sarcoma u. a. m. "
Thus the word formation morpheme “-om” was secondarily filled with lexical meaning by the medical terminologists and the stage reached in which an original morpheme assumes the rank of a lexeme, an appellative .
The literal translation of the medizinterminologischen Art word 'Myel-om', English "myeloma a " is the German "so bone marrow tumor ," bone marrow cancer.
Word history
“The term 'multiple myeloma' was introduced by von Rustitzky in 1873 when he found eight separate tumors of bone marrow and designated them as 'multiple myelomas'. In Russia, the term Rustitzky's desease is often used. "
“ The term 'multiple myeloma' was introduced by Rustitzky in 1873 when he found eight separate bone marrow tumors. He called them 'multiple myelomas'. Rustitzky's disease is widely known in Russia . "
In order to characterize the attachment of tumors to the bone marrow, the surgeon J. von Rustitzky introduced the term myeloma into medical terminology in 1873 after the autopsy of the 47-year-old servant Joh. Kessler .
"The microscopic as well as macroscopic nature of the above tumors entitles us to classify them as a special class and to give them the name 'myelomas' in order to denote the identity of their structure with the bone marrow."
At that time, around 1873, Rustitzky interpreted this disease, "the causal factor of which remains unknown to us", as a benign, local hypertrophication of the bone marrow:
"The fact that the tumors are tied to the bone marrow also justifies the claim that the tumors present, although multiple, were not malignant in the strict sense ... Rather, it seems more advisable to classify these tumors as other benign but multiple tumors ( adenomas , lipomas , Fibromas , exostoses etc.) and to consider a local disease of the bone marrow, as an interpretation of the tumor formation, and to designate this disease, the causal factor of which we do not know, as hypertrophication of the bone marrow tissue . "
In the years that followed, the titles of specialist articles show how the term multiple myeloma proposed by Rustitzky became an accepted terminological common knowledge, and how slowly advancing medical research gradually shed more light on the "unknown causal factor behind this disease".
Hippocrates started this slow process in the history of science in the 5th century BC. BC. In a nutshell by an aphorism : Ars longa, vita brevis , loosely translated: the healing art advances slowly and life is short.
Medical history

Multiple myeloma is not a phenomenon of modern times, people have been afflicted by this disease for many centuries:
“The working group of A. Zink from the Pathological Institute of the LMU Munich examined 415 Egyptian mummies (1500–500 BC) in the context of paleopathology . Four of these showed malignant skeletal changes, two mummies had multiple osteolyses on the spine, pelvis and skull suspected of being myeloma . "
Also bone finds from the 2nd century BC. BC have the typical destructive characteristics of the disease.
Historical case report: Thomas McBean (1850):
The first well-documented case of multiple myeloma comes from a case report by Macintyre. He described the medical history of the English general merchant Thomas A. McBean, who presented himself to his doctor's office in 1845 at the age of 45. McBean complained that something was wrong with his urine - he had frequent urges to urinate and "urine made his garment stiff" even though he had not noticed any discharge from the urethra . In addition, the patient suffered from unusual weakness and emaciation, during a walk he then had the feeling "something in the chest cracked or gave way", and McBean fell and "could not get up for a few minutes because of severe pain". Doctor Macintyre treated the patient with a bandage of the chest ("strengthening plasters") and prescribed physical restraint. A month later, the patient was again in severe pain and repeated blood-letting , leeches, and cupping cones were used, but this did not bring lasting relief, so McBean referred to another doctor, Dr. Watson, introduced. He began treatment with iron and quinine , which led to an amazing improvement that lasted six months. In October 1845, however, the patient suffered severe pain in the spine and sciatica , which did not improve even with the use of warm baths, camphor powder and ointment. Dr. Macintyre also diagnosed edema on McBean's body and therefore examined the patient's urine. This was dark and flocculated when heated (“abound in animal matter”). Almost at the same time, Watson sent a urine sample to doctor and chemist Bence Jones with the question “What is it?” Who identified proteins in the urine of McBean - and other patients with similar symptoms - and characterized them. McBean's condition deteriorated rapidly through 1845, in severe pain and unable to get out of bed. McBean finally died on January 1, 1846, and his death certificate recorded " atrophy due to albuminuria " as the cause of death.
The autopsy, attended by Doctors Macintyre, Watson and Jones, showed bones that "were easy to cut with a knife and simply broke." The ribs literally crumbled and contained a blood-red, gelatinous, and oily mass. The entire spine was also similar. The pelvis, humerus and thigh bones "withstood any attempt to break them by hand". The heart, lungs and liver were described as largely normal. John Dalrymple , surgeon and member of the Microscopic Society, examined two lumbar vertebrae and a McBean rib. He found holes in the patient's bone, which were filled with a red, gel-like mass. He examined these under the microscope and found large, uniform-looking round to oval cells, some with several nuclei . The woodcuts made from drawings by Dalrymple show the criteria for myeloma cells that are still valid today.
Epidemiology
The incidence of multiple myeloma is around four to six new cases / 100,000 people per year. This corresponds to ten percent of all haematological cancers or one percent of all cancers as a whole. It is a disease of older age, with the median age at the time of diagnosis being 66 years. Only two percent of the patients are younger than 40 years. It is very rare in children. Men are slightly more likely to be affected than women, and Afro-Americans are about twice as likely as the white US population. According to current data, the five-year prevalence worldwide for all age groups is over 350,000 people.
Depending on the antibody formed, a distinction is made between the following types:
| Monoclonal tumor product | frequency | IgG | IgA | IgM | IgD | IgE | Kappa | Lambda | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Exclusively intact immunoglobulin | 5-10% | approx. 55% * | approx. 20% * | approx. 0.5% * | approx. 2% * | extremely rare | ||||
| Free light chain with or without intact immunoglobulin | 90-95% | Free light chain with intact immunoglobulin | 70-75% | 57% * | 34% * | |||||
| Exclusively free light chain | 15-20% | |||||||||
| Non-secretory | 1-2% | |||||||||
| * based on all myeloma patients | ||||||||||
It should be emphasized that in a myeloma in which an intact immunoglobulin is formed, e.g. B. in IgG myeloma, in approx. 90-95% of cases there is also an increased amount of a free light chain (e.g. a free light chain of type κ in IgGκ myeloma). Only an intact immunoglobulin is found in only 5–10% of cases and in a few cases the plasma cells do not secrete any monoclonal immunoglobulin or free light chains. In these cases one speaks of a so-called non-secretory multiple myeloma. Monoclonal immunoglobulins are partially produced, but then remain within the plasma cell and can only be detected by immunohistochemical methods (intracellular staining). In addition, there are seldom cases in which the plasma cells produce neither intact immunoglobulins nor free light chains (so-called true non-secretory multiple myeloma).
Pathogenesis
Risk factors
The pathogenetic causes of multiple myeloma are the subject of current research. Influences of various environmental factors are discussed. Ionizing radiation, herbicides (e.g. glyphosate ), obesity, autoimmune diseases and increased inflammatory processes and infections are associated with the development of the disease . In some cases, various genetic translocations have been described, but their influence has not yet been fully clarified. A connection with conventional gas production or emissions resulting from fracking is also suspected.
development
The transition from monoclonal gammopathy of unclear significance (MGUS) to symptomatic myeloma proceeds in several steps and can take a few months to several decades. The malignant degeneration of the myeloma cells usually takes place outside the bone marrow in the germinal centers of peripheral lymphatic organs . The B cells that enter these germinal centers have already completed the first differentiation steps ( V (D) J recombination ). It is at this stage that the genetic changes occur that ultimately lead to the development of multiple myeloma.
In most patients, translocations are observed that cause an oncogene to come under the control of a regulatory gene and thereby strongly activate expression. In multiple myeloma this is most often (approx. 80%) the immunoglobulin enhancer gene on chromosome 14 gene locus q31. Frequent partners in this translocation are parts of chromosomes 4 (4p16.3; Fibroblast Growth Factor Receptor ), 6 (6p21; Cyclin D3 ), 11 ( Bcl-1 , Cycin D1 ), 16 (16q23; C-maf ) and 20 (20p11 ; maf8 ). It is rare to find 8q24 ( c-myc ) and even more rarely 18q21 ( bcl-2 ), 11q23 (MLL-1) and 20q11 ( maf B ). Other genetic changes occur as the disease progresses. The various genetic changes go hand in hand with different disease courses and may define their own entities . By detecting certain genetic changes in a patient's myeloma cells, statements about the prognosis can be made in some cases. For example, it is known that a deletion (del13q) or a monosomy of chromosome 13 is associated with a shorter survival time on average. After the clonal reproduction of a degenerate plasma cell , infiltration of the bone marrow occurs and the like. U. also with accompanying destruction of the bone and displacement of normal hematopoiesis (blood formation). The malignant cells secrete growth factors and cytokines that activate the osteoclasts (including OAF ), which ultimately leads to bone resorption. The antibodies or antibody parts (free light chains or fragments of the heavy chain ) formed by the malignant cells , which accumulate in the body, are responsible for some symptoms and complications of the disease.
Symptoms
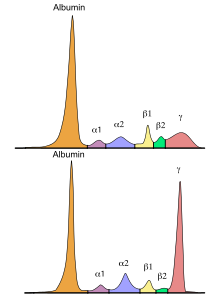
Some patients are diagnosed with the disease by chance from a blood test using abnormal serum protein electrophoresis . However, most patients experience symptoms and complications:
- Nonspecific symptoms such as weakness, fatigue, and weight loss
- Overall, around 60% of patients develop bone changes:
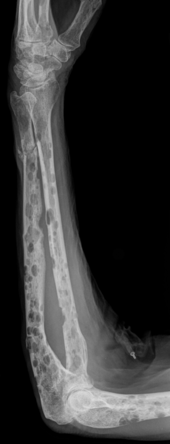
- Severe bone loss / damage due to osteolysis (in contrast to bone metastases, the damage looks like punched out - typical: shotgun skull)
- Secondary osteoporosis caused by osteolysis (not to be confused with the primary form)
- Bone pain and fractures from osteolysis
- Decrease in bone marrow function ( bone marrow insufficiency ) with anemia and a tendency to bleeding and a decrease in white blood cells ( leukopenia ) and / or platelets ( thrombocytopenia ) with a tendency to thromboembolism
- Susceptibility to infection due to a lack of polyclonal antibodies and a lack of white blood cells (leukopenia)
- Increased viscosity of the serum due to monoclonal immunoglobulins can lead to headache, drowsiness, dizziness, nystagmus , hearing and visual disturbances, drowsiness, coma and seizures ( hyperviscosity syndrome )
- Under certain circumstances deposits of monoclonal immunoglobulins or free light chains in the peripheral nervous system ( polyneuropathy )
-
Chronic or acute kidney failure . If the impairment of kidney function is due to a monoclonal free light chain or a monoclonal intact immunoglobulin, then monoclonal gammopathy can be said to be of renal significance . Examples are (selection):
- Cast nephropathy : In the presence of uromodulin , the free light chains in the kidney tubules can precipitate in the form of protein cylinders. These precipitates have a direct toxic effect on the cells of the kidney tubules and can lead to a rapid loss of kidney function (acute kidney failure) (classic myeloma kidney ; also known as cast nephropathy).
- Light chain deposition disease : Deposition of light chains in the basement membranes of kidney corpuscles and tubules can lead to light chain deposition disease. This manifests itself in a greatly increased excretion of protein in the urine ( nephrotic syndrome ) and leads to chronic kidney failure.
- Fanconi syndrome : The light chains can lead to dysfunction of the cells in the main part of the kidney tubules. These functional disorders manifest themselves in a reduced excretion of acids ( acidosis ) and an increased loss of phosphate , glucose , uric acid and amino acids .
- AL amyloidosis : The misfolding of free light chains leads to the formation of fibrils , which are deposited in the form of amyloids in kidney tissue and kidney vessels. As with light chain deposition disease, the result is increased proteinuria and chronic kidney failure.
The typical but also unspecific symptoms make a clear diagnosis difficult. Many of the symptoms described here can also occur at an earlier stage of the disease and do not necessarily have to develop in what is by definition multiple myeloma. A comprehensive anamnesis of the patient enables a reliable diagnosis of the underlying disease. Patients with known pre-existing conditions such as osteoporosis or polyneuropathy are more likely to have monoclonal gammopathy such as multiple myeloma. The next section summarizes the currently valid recommendations for diagnosis and the diagnostic criteria.
diagnosis
Diagnostic criteria
The basic requirement is a ≥10% infiltration of the bone marrow by clonal plasma cells or an extramedullary plasmacytoma confirmed by biopsy. If one or more of the following criteria are also met, the diagnosis of multiple myeloma can be made:
-
CRAB criteria : C alcium, R enale insufficiency, A Naemie, B one = Bone
- Hypercalcaemia in the serum detectable with a value of> 2.75 mmol / l or> 0.25 mmol / l above the upper normal value.
- Renal insufficiency , defined as serum creatinine> 2 mg / dl or creatinine clearance <40 ml / min.
- Anemia , defined as a hemoglobin value of <10 g / dL or more than 2.0 g / dL below normal.
- Bone lesions with at least one osteolytic lesion, detectable by X-ray, CT or PET-CT.
-
SLIM criteria : S ixty Percent = 60 percent, Li ght chain ratio , M RT-lesion
- ≥ 60% infiltration of plasma cells in the bone marrow.
- a free-light chain ratio of the tumor-associated free light chain (involved free light chain) to the non-tumor-associated free light chain (not involved free light chain) of ≥ 100 (the tumor-associated free light chain must be present at a concentration of at least 100 mg / l).
- > 1 focal bone lesion with a size of at least 5 mm detected by a whole body image in the MRI.
Differential diagnosis
In order to be able to make the diagnosis based on the above criteria, various examinations must be carried out. The following methods are mentioned in the hematological guidelines:
| 1. Laboratory diagnostics | 2. Imaging | 3. Examination of the bone marrow |
|---|---|---|
|
The standard methods are:
Also:
|
|
|
If the above-mentioned examinations result in a positive result or if the CRAB / SLiM criteria are met, the following additional examinations must be considered before initiating therapy:
- Magnetic resonance tomography (MRI) of targeted regions (possibly with contrast agent), especially in
- neurological symptoms with suspected spinal cord compression
- Suspected extramedullary manifestations
- Echocardiography (especially cardiac amyloidosis)
In addition to reactive bone marrow changes, the preliminary stages SMM and MGUS must also be differentiated from a differential diagnosis; see the general diagnostic criteria of monoclonal gammopathies .
forecast
Many different factors play a role in the development of the disease and in its course. So z. B. the age at the beginning of the illness, the general physical condition, the extent of the accompanying illnesses, the occurrence of complications or unforeseen events and / or the response to the treatment measures. The survival rates have been within the last decade, according to Hartmut Goldschmidt (s. U.) Of the new therapeutic options significantly improved. You can extend overall survival and the symptom-free time . Last but not least, they increase the quality of life of those affected and their relatives. In summary, according to the current status, an absolute five-year survival rate of 41% for men and 40% for women can be spoken of. Taking into account mortality in the general population, the relative five-year survival rate is currently 48% for men and 45% for women. For men and women, the relative ten-year survival rates are 31% and 30%, respectively. A division into stages and risk stratification allow patients to be assessed in a more differentiated manner and a more precise prognosis can be made.
Staging
The Brian Durie and Sydney Salmon classification, which is still common, distinguishes three stages:
| stage | features |
|---|---|
| Stage I. |
|
| Stage II | between stage I and III |
| Stage III | at least one of the following criteria:
|
| Addition A B |
|
The classification according to the International Staging System (ISS) is newer , in which only β 2 -microglobulin and albumin are taken into account.
| stage | features |
|---|---|
| ISS I | β 2 -microglobulin <3.5 mg / l and albumin ≥ 35 g / l |
| ISS II | β 2 -microglobulin <3.5 mg / l and albumin <35 g / l or β 2 -microglobulin 3.5–5.5 mg / l |
| ISS III | β 2 -microglobulin> 5.5 mg / l |
Risk stratification
If the preliminary stage MGUS was diagnosed before the disease developed, the following factors can be used to assess the risk of developing symptomatic multiple myeloma:
- abnormal ratio of free light chains
- M protein in serum> 15 g / l
- M protein of the IgA, IgM, IgD or IgE type
Taking into account the average life expectancy, a 2% risk in the absence of all factors, a 10% risk in the case of evidence of one factor, an 18% risk in the event of evidence of two factors and a 27% risk in the event of all three Factors for the further development of MGUS to multiple myeloma within 20 years can be assumed. If the affected patients are then regularly examined (depending on the risk classification every six months, annually or every two to three years), the risk of complications due to the progression of the disease or its preliminary stages can be reduced.
In a similar way, patients with already proven smoldering multiple myeloma can be classified for an approx. 50% risk of progression within two years based on the following factors. The basic requirement is at least 10% clonal plasma cells in the bone marrow and one or more of the following criteria:
- Increase in serum M protein by at least 25% in two consecutive measurements
- M protein in serum of at least 30 g / l
- Ratio of tumor-associated to non-tumor-associated free light chain at least 8
- IgA type SMM
- Suppression of two non-tumor-associated immunoglobulin isotypes
- Increased number of circulating plasma cells
- Cytogenetic abnormalities on chromosomes 4, 14 and 17
- Detection by PET-CT or MRI of focal lesions (at least one) without osteolytic bone damage
- 50–60% clonal plasma cells in bone marrow
An abnormal free light chain ratio, an M protein in serum of at least 30 g / l and clonal plasma cells in the bone marrow of at least 10% are considered independent risk factors. After five years, the risk of progression in the presence of one of these factors is 25%, in the presence of two factors 51% and in the presence of all three factors 76%. The recommended follow-up intervals are every two to three months, every four to six months if the values are stable in the first year after diagnosis, and then every six to twelve months if the values remain stable.
therapy
Up to the present state of science, a cure for multiple myeloma is not possible. Regular laboratory tests as well as X-ray and bone marrow examinations provide information about the progression of the disease. Therapy can be initiated if end organ damage (CRAB and SLiM criteria - see above ) is evident . There are now numerous treatment options that can keep the disease level stable over a longer period of time, reduce the symptoms and significantly increase the quality of life for those affected.
Therapy of the primary disease
Affected people can, depending on the possibility and indication , be treated with chemotherapy , drugs that affect the immune system ( immunomodulators ) and drugs that inhibit bone breakdown ( bisphosphonates ) and receive a bone marrow transplant . Survival rates have improved significantly over the past decade with new drugs such as bortezomib , carfilzomib , ixazomib , lenalidomide , panobinostat , pomalidomide and thalidomide . The primary therapy is always determined individually for each patient. Very significantly influences z. B. the age the therapy decision. The general physical condition and the extent of the accompanying illnesses also play a major role in determining which therapy can be considered suitable. Theoretically, the following options are possible, whereby a higher age z. B. excludes a stem cell transplant:
chemotherapy
The classic combination therapy consists of the cytostatic drug melphalan and the cortisone preparation prednisone . The chemotherapy usually takes place in cycles. Known and feared side effects are hair loss and nausea. However, these side effects can now be alleviated or avoided entirely with effective medication. Another chemotherapeutic agent is bendamustine , which is characterized by a more favorable side effect profile.
In 2019, bortezomib , lenalidomide and thalidomide were also evaluated in various combinations for the treatment of multiple myeloma. Depending on the combination, a sometimes strong increase in overall survival can probably be achieved.
Autologous stem cell transplant
This involves transplanting your own stem cells . Stem cells are obtained from the patient's bone marrow and returned to the patient after chemotherapy. These stem cells usually lead to the regeneration of blood formation within a short time.
Allogeneic stem cell transplant
This much rarer method involves transplanting stem cells from a foreign donor. For this procedure, the patient must meet certain requirements. The hematopoietic system of the recipient is permanently destroyed in order to create a new hematopoietic system that is free of diseased blood cells by giving blood stem cells that are as identical as possible. In contrast to autologous transmission, there is a fundamental risk of the graft-versus-host reaction ( graft-versus-host disease ), but this phenomenon can also have the positive effect of the graft-versus-malignancy reaction ( graft-versus-malignancy Effect ). Patients must take medication to suppress the risk of rejection ( immunosuppressants ) for up to a year . Besides cornea transplantation, allogeneic stem cell transplantation is the only "organ transplantation" in which the immunosuppressants do not have to be taken for life.
The use of mesenchymal stromal cells was evaluated to treat or avoid graft-versus-host reactions. However, mesenchymal stromal cells cause little or no change in all-cause mortality, return of malignancy, and the incidence of acute and chronic graft-versus-host reaction for prophylactic purposes.
radiotherapy
In contrast to chemotherapy, radiation therapy works locally. With their help, the ability of the malignant cells to divide is to be stopped and thus the further growth of the tumor is prevented. Side effects occur locally, depending on the location, e.g. B. reddening of the skin, diarrhea or nausea / vomiting occur.
Immune modulation
Immunomodulatory substances (IMiDs for the English term: Immunomodulatory Imid Drugs) have proven to be extremely effective against cancer in recent years. The release of inflammatory or tumor-promoting substances is inhibited. IMiDs include: lenalidomide (trade name: Revlimid) and thalidomide ; Pomalidomide is on the market in the USA under the trade name Pomalyst. In Europe, pomalidomide was approved under the trade name Imnovid in August 2013.
In contrast to chemotherapy , for example , in which, in addition to destroying the tumor tissue, temporary impairment of healthy cells is inevitable, IMiDs work specifically against the tumor-causing processes. In July 2007, the European Medicines Agency (EMA) approved the oral lenalidomide dosage form (Revlimid hard capsules, Celgene ) in combination with dexamethasone for the treatment of patients who had received at least one previous therapy . It has been classified as a medicine used in rare diseases ( orphan medicine ). In combination therapy with dexamethasone , it is more effective than monotherapy with dexamethasone in relapses or therapy failure. In addition to its immunomodulating effect, lenalidomide has other tumor control mechanisms:
- Activation of immune cells (T cells and natural killer cells, which in turn attack the tumor cells)
- Growth stop through direct attack on the tumor cells
- Angiogenesis inhibition (inhibition of the formation of new blood vessels that supply the tumor with nutrients)
- Suppression of the release of tumor-promoting messenger substances
- Apoptosis by cell cycle -Arrest
- Inhibits osteoclast activation
Proteasome inhibition
Proteasomes are protein complexes that regulate the growth and death of cells ( apoptosis ). Proteasome inhibitors are substances that inhibit the activity of proteasomes. They slow down cell growth and accelerate apoptosis. The first proteasome inhibitor to be approved in both the US and the EU is bortezomib (trade name: Velcade); Carfilzomib (trade name: Kyprolis) was approved in both the US and the EU in 2015. Ixazomib has been approved in the EU since 2016. As an enhancer of proteasome inhibitors, the histone deacetylase inhibitor panobinostat (trade name: Farydak) has been approved in the EU since 2015 for the treatment of multiple myeloma under certain conditions.
Biologics
Recent research has led to the development of highly specific biologics that have been approved for the treatment of multiple myeloma for some time. These include the monoclonal antibodies daratumumab and elotuzumab . While daratumumab binds specifically to the glycoprotein CD38, which is overexpressed on myeloma cells , which triggers apoptosis of the cells, elotuzumab develops its immunostimulatory properties through interaction with the surface protein SLAMF7 on myeloma cells.
Supportive therapy
Supplementary (so-called supportive) therapeutic measures can help to contain accompanying symptoms and prevent complications. Therefore, it was evaluated whether physical activity as a supplement to standard therapy has advantages for the patients. The evidence is very uncertain about the effect of exercise on anxiety and major adverse events. Exercise may cause little or no change in mortality, quality of life, and physical function. Exercise may cause a small reduction in depression.
process control
The effectiveness of a therapy can be assessed on the basis of various parameters. The currently valid definitions from international and national guidelines that are used here are listed below.
Remission criteria
| Criterion / status | M protein in SPE | M protein in IFE | Free light chains | Manifestation in soft tissues | Percentage of plasma cells in the bone marrow |
|---|---|---|---|---|---|
| stringent complete remission (sCR) | not detectable in serum and urine | normalized ratio of the free light chains | none detectable | ≤ 5%; no clonal plasma cells detectable by immunohistochemistry | |
| complete remission (CR) | not detectable in serum and urine | none detectable | ≤ 5% | ||
| very good partial remission (VGPR) | at least 90% reduction in serum and less than 100 mg / 24h in urine or no M protein detectable in serum and urine | verifiable | |||
| partial remission (PR) | at least 50% decrease in the serum and at least 90% reduction in the urine or less than 200 mg / 24h in the urine | if M protein cannot be determined,> 50% reduction in the difference between tumor-associated (involved) and non-tumor-associated (not involved) free light chain = dFLC | > 50% reduction (mandatory criterion) | if the proportion before therapy is more than 30% and if the M protein and free light chain ratio cannot be determined, the infiltration rate is reduced by more than 50% | |
| stable disease (SD) | none of the criteria met | ||||
| progressive disease (PD) | at least 25% increase in serum and absolute at least 0.5 g / dl and / or at least 25% increase in urine or absolute ≥ 200 mg / 24h | at least 25% increase in dFLC in the serum, absolute by at least 100 mg | Progress or new appearance | > 25% increase in relation to the value at the time of the best response and in absolute terms at least 10% | |
Minimal residual disease (MRD)
In a large majority of patients, even after a complete remission has been achieved using various methods, minimal residual disease (MRD) can be detected. In such a case, the patient is not to be regarded as cured and, under certain circumstances, a relapse may occur later . In the meantime established methods for the detection of a residual disease are next-generation sequencing , flow cytometry as well as MRI and PET. More recent studies also show that the serological determination of the immunoglobulins, according to their bound light chain, allows statements to be made about a possibly underlying minimal residual disease - e.g. B. IgGκ as M protein in the normal range and IgGλ as normal (polyclonal) immunoglobulin below the normal range as an indication of an MRD. If MRD negativity is proven, a longer progression-free and overall survival can be assumed. These methods are currently not yet standard and do not yet make a predictive contribution to further therapy decisions.
literature
- MA Bärtsch Current aspects in the diagnosis and therapy of plasma cell myeloma . In: Deutsches Ärzteblatt 2017, 142 (11), pp. 800–804.
- D. Felsenberg Monoclonal Gammopathy of Unclear Significance (MGUS) . In: Forum Sanitas 2018, 2nd edition, pp. 39–41.
- Hartmut Goldschmidt : Multiple myeloma (plasmacytoma). Diagnosis and therapy . 2nd Edition. Unimed, Bremen 2011, ISBN 978-3-8374-1032-7 .
- Robert A. Kyle, David P. Steensma: Multiple Myeloma: A History. In: James S. Malpas, Daniel E. Bergsagel, Robert E. Kyle: Myeloma: Biology and Management. Saunders, 3rd Edition, 2004, pp. 99-117, ISBN 978-0-7216-0006-2 .
- KM Kortüm Multiple myeloma . In: Der Internist 2013, 54 (8), pp. 963–977.
- J. v. Rustitzky: Multiple Myeloma. In: Deutsche Zeitschrift für Chirurgie, 3, 1873, pp. 162-172, article in full text - on the server of the Bayerische Staatsbibliothek.
- Christian Straka, Hermann Dietzfelbinger (eds.): MANUAL Multiple Myelom. Recommendations for diagnosis, therapy and follow-up care. Tumor Center Munich, W. Zuckschwerdt Verlag, 5th revised edition, Munich 2017, ISBN 978-3-86371-211-2 .
Web links
- Myeloma Germany e. V.
- AMM online. Network for myeloma patients, information portal with patient forum
- Official site of the International Myeloma Working Group (English)
- Plasmacytoma / Multiple Myeloma. Reply. Aid. Perspectives. (PDF; 1.17 MB) Blue Guide # 22 of the German Cancer Aid
- Myeloma Contact Group Switzerland
- Multiple myeloma , pathology - Image database Pathopic of the University of Basel; PathoPic - Instructions (PDF; 2.2 MB)
- Multiple Myeloma - A Cancer Disease of the Bone Marrow - Patient Handbook (German edition) of the International Myeloma Foundation
- Multiple Myeloma - A cancer of the bone marrow - Brief summary of the disease and therapy options (German-language edition) of the International Myeloma Foundation
- Understanding Multiple Myeloma - A Cancer of the Bone Marrow - Freelite® and Hevylite® Serum Assays. (German language edition) of the International Myeloma Foundation
- wikilite.com - English-language information site on the biological background, diagnostics, follow-up and therapy of monoclonal gammopathies
- The Myeloma Beacon English-language site with current information on multiple myeloma
- Surgical therapy need for multiple myeloma
Individual evidence
- ↑ Ludwig Heilmeyer , Herbert Begemann: Blood and blood diseases. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition ibid. 1961, pp. 376-449, here: pp. 432-434: Das Myelom (Kahler's disease or myelogenous plasmocytoma) .
-
↑
"To use a proper name to refer to MM as Kahler's disease or Bozzolo's disease is unusual internationally."
- Hartmut Goldschmidt : Multiple myeloma (plasmacytoma) diagnosis and therapy. 2nd Edition. Uni-Med Verlag, Bremen 2011, ISBN 978-3-8374-1032-7 , p. 10. - ↑ Classification according to the ICD code
- ↑ O. Landgren et al .: Monoclonal gammopathy of undetermined significance (mgus) consistently precedes multiple myeloma: a prospective study . In: Blood . 113, No. 22, 2009, pp. 5412-5417. doi : 10.1182 / blood-2008-12-194241 . PMID 19179464 .
- ↑ HT Tsai et al .: Evidence of serum immunoglobulin abnormalities up to 9.8 years before diagnosis of chronic lymphocytic leukemia: a prospective study . In: Blood . 114, No. 24, 2009, pp. 4928-4932. doi : 10.1182 / blood-2009-08-237651 . PMID 19828698 .
- ↑ a b c d e N. van de Donk et al .: The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network . In: Haematologica . 99, No. 6, March 21, 2014, pp. 984-96. doi : 10.3324 / haematol.2013.100552 . PMID 23224402 . PMC 4040895 (free full text).
- ^ SV Rajkumar et al .: Smoldering multiple myeloma . In: Blood . 125, No. 20, April 2, 2015, pp. 3069-75. doi : 10.1182 / blood-2014-09-568899 . PMID 25838344 . PMC 4432003 (free full text).
- ↑ SH Swerdlow, E. Campo, NL Harris, ES Jaffe, SA Pileri, H. Stein , J. Thiele, JW Vardiman (eds.): WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th edition. IARC Press, Lyon 2008, ISBN 978-92-832-2431-0 (the 2008 WHO classification)
- ↑ Hartmut Goldschmidt : The multiple myeloma (plasmacytoma) diagnosis and therapy . 2nd Edition. Uni-Med Verlag, Bremen 2011, ISBN 978-3-8374-1032-7 , p. 10.
- ↑ ICD-10 classification of the WHO (Version 2019) - C90.01 and C90.03
- ↑ a b N Leung et al .: Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant . In: Blood . 120, No. 22, November 22, 2012, pp. 4292-4295. doi : 10.1182 / blood-2012-07-445304 . PMID 23224402 .
- ↑ O. Landgren et al .: Shall we treat smoldering multiple myeloma in the near future? . In: Hematology Am Soc Hematol Educ Program . 2017, No. 1, 2017, pp. 194–204. doi : 10.1182 / asheducation-2017.1.194 . PMID 29222256 .
- ↑ Oswald Panagl : The suffix -om (= Greek -ωμα) - a pseudo-lexeme of medical terminology. In: Glotta , Volume 49. 1./2. Heft, 1971, pp. 42-45, Vandenhoeck & Ruprecht, JSTOR 40266190 .
- ↑ Oswald Panagl : The suffix -om (= Greek -ωμα) - a pseudo-lexeme of medical terminology. In: Glotta , Volume 49. 1./2. Heft, 1971, p. 44, Vandenhoeck & Ruprecht, JSTOR 40266190 .
- ^ Robert A. Kyle, David P. Steensma: Multiple Myeloma: A History . In: James S. Malpas, Daniel E. Bergsagel, Robert E. Kyle: Myeloma: Biology and Management. Saunders, 3rd edition, 2004, p. 111.
- ↑ Hartmut Goldschmidt : "The multiple myeloma (plasmacytoma) diagnosis and therapy." 2nd edition. Uni-Med Verlag, Bremen 2011, ISBN 978-3-8374-1032-7 , p. 11.
- ↑ J. v. Rustitzky: Multiple Myeloma. In: Deutsche Zeitschrift für Chirurgie, 3, 1873, p. 170.
- ↑ J. v. Rustitzky: Multiple Myeloma. In: Deutsche Zeitschrift für Chirurgie, 3, 1873, p. 171: Myelom & language = de & c = default full text article - on the server of the Bavarian State Library.
- ↑ J. v. Rustitzky: Multiple Myeloma. In: Deutsche Zeitschrift für Chirurgie, 3, 1873, p. 171.
- ↑ see the bibliography at the end of the previously cited article by Robert A. Kyle, David P. Steensma: Multiple Myeloma: A History , 2004, pages 114-116.
- ↑ J. Dalrymple: On the microscopic character of mollities ossium . In: Dublin Quarterly Journal of Medical Science . No. 2 , 1846, p. 85-95 .
- ↑ A. Nerlich, H. Rohrbach, A. Zink: Paleopathology of ancient Egyptian mummies and skeletons. In: Pathologist. 23, 2002, p. 379, doi: 10.1007 / s00292-002-0558-9
- ^ Christian Straka, Hermann Dietzfelbinger (editor): Manual Multiple Myelom. Recommendations for diagnosis, therapy and follow-up care. Tumor Center Munich, W. Zuckschwerdt Verlag, 5th revised edition 2017, ISBN 978-3-86371-211-2 .
- ^ D. Morse, RC Dailey, J. Bunn: Prehistoric multiple myeloma . In: Bulletin of the New York Academy of Medicine . No. 50 , 1974, pp. 447-458 , PMID 4594853 .
- ↑ a b c W Macintyre: Case of mollities and fragilitas ossium, accompanied with urine strongly charged with animal matter . In: Medical and Surgical Transactions of London . No. 33 , 1850, pp. 211-232 .
- ^ A b c R. A. Kyle: Multiple myeloma: an odyssey of discovery . In: British Journal of Hematology . No. 111 (4) , 2000, pp. 1035-1044 , PMID 11167737 .
- ↑ H. Bence Jones: On a new substance occurring in the urine of a patient with mollities ossium . In: Philosophical Transactions of the Royal Society of London (Biology) . 1848, p. 55-62 .
- ↑ a b WHO Disease and Injury Country Estimates . In: World Health Organization . 2009. Archived from the original on November 11, 2009. Retrieved on November 11, 2009.
- ↑ Federal Statistical Office for Multiple Myeloma PDF, 216 KB, accessed on April 11, 2017
- ↑ JL Harousseau, P. Moreau: Autologous hematopoietic stem-cell transplantation for multiple myeloma. In: The New England Journal of Medicine . Volume 360, Number 25, June 2009, pp. 2645-2654, ISSN 1533-4406 . doi: 10.1056 / NEJMct0805626 . PMID 19535803 . (Review).
- ^ SC Bernstein, AR Perez-Atayde, HJ Weinstein: Multiple myeloma in a child. In: Cancer. Volume 56, Number 8, October 1985, pp. 2143-2147, ISSN 0008-543X . PMID 3928137 .
- ↑ Fact Sheet of the International Agency for Research on Cancer WHO GLOBOCAN 2012
- ↑ a b G. P. Mead et al .: Serum free light chains for monitoring multiple myeloma . In: Br J Hematol . 126, No. 3, 2004, pp. 348-354. doi : 10.1111 / j.1365-2141.2004.05045.x . PMID 15257706 .
- ^ RA Kyle et al .: Review of 1027 patients with newly diagnosed multiple myeloma . In: Mayo Clin Proc . 78, No. 1, 2003, pp. 21-33. doi : 10.4065 / 78.1.21 . PMID 12528874 .
- ↑ M. Drayson et al .: Effects of paraprotein heavy and light chain types and free light chain load on survival in myeloma: an analysis of patients receiving conventional-dose chemotherapy in Medical Research Council UK multiple myeloma trials . In: Blood . 108, No. 6, 2006, pp. 2013-9. doi : 10.1182 / blood-2006-03-008953 . PMID 16728700 .
- ↑ MM Dupuis et al .: Non-secretory multiple myeloma: from biology to clinical management . In: Onco Targets Ther . 9, 2004, pp. 7583-7590. doi : 10.2147 / OTT.S122241 . PMID 28008276 . PMC 5171196 (free full text).
- ↑ Lower Saxony Cancer Register - Special evaluation of the Bothel community 2014 (PDF)
- ↑ Seema Singhal, Jayesh Mehta: Multiple Myeloma. In: Clin J Am Soc Nephrol . 2006, 1, pp. 1322-1330.
- ^ AM Rajan et al .: Interpretation of cytogenetic results in multiple myeloma for clinical practice . In: Blood Cancer J . 5, No. 10, October 30, 2015, p. E365. doi : 10.1038 / bcj.2015.92 . PMID 26517360 . PMC 4635200 (free full text).
- ↑ SK Kumar et al .: Multiple myeloma . In: Nat Rev Dis Primers . 3, July 20, 2017, p. 17046. doi : 10.1038 / nrdp.2017.46 . PMID 28726797 .
- ↑ a b c DGHO guideline "Multiple Myeloma" . Retrieved June 25, 2018.
- ↑ Laura M. Dember: Light Chains, Casts, Sheets and Fibrils: Monoclonal Immunoglobulin Diseases and Immunotactoid / Fibrillary Glomerulopathy. In: Clin J Am Soc Nephrol. 2006, 1, pp. 1320-1321.
- ↑ T Golombick et al .: Prevalence of monoclonal gammopathy of undetermined significance / myeloma in patients with acute osteoporotic vertebral fractures . In: Acta Haematol . 120, No. 2, October 14, 2008, pp. 87-90. doi : 10.1159 / 000162282 . PMID 18852483 .
- ^ N Steiner et al .: Are neurological complications of monoclonal gammopathy of undetermined significance underestimated? . In: Oncotarget . 8, No. 3, December 10, 2016, pp. 5081-5091. doi : 10.18632 / oncotarget.13861 . PMID 27974705 . PMC 5354894 (free full text).
- ^ A b SV Rajkumar et al .: International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma . In: Lancet Oncology . November 15, 2014, pp. E538 – e548. doi : 10.1038 / leu.2010.60 . PMID 20410922 .
- ↑ Erentraud Hömberg: News from the Myeloma World Congress: Both diagnostics and therapy are developing rapidly. Medscape, April 23, 2013, accessed December 28, 2015 .
- ↑ a b c S. K. Kumar et al. a .: Improved survival in multiple myeloma and the impact of novel therapies . In: Blood . No. 111 (5) , March 2008, pp. 2516-2520 , doi : 10.1182 / blood-2007-10-116129 .
- ^ BG Durie et al .: A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. . In: Cancer . 36, No. 3, September 1975, pp. 842-854. PMID 1182674 .
- ^ A. Palumbo et al .: Revised International Staging System for Multiple Myeloma: a report from IMWG . In: J Clin Oncol . 33, No. 26, September 10, 2015, pp. 2863–2869. doi : 10.1200 / JCO.2015.61.2267 . PMID 26240224 . PMC 4846284 (free full text).
- ↑ a b c R. A. Kyle et al .: Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management . In: Leukemia . 24, February 4, 2010, pp. 1121-1127. doi : 10.1038 / leu.2010.60 . PMID 20410922 .
- ↑ RS Go et al .: Determining the clinical significance of monoclonal gammopathy of undetermined significance: a SEER-Medicare population analysis . In: Leukemia . 15, No. 3, September 28, 2014, pp. 177-186.e4. doi : 10.1016 / j.clml.2014.09.004 . PMID 25445471 . PMC 4344843 (free full text).
- ↑ International Myeloma Working Group (IMWG) Criteria for the Diagnosis of Multiple Myeloma ( Memento of the original dated November 7, 2017 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b M. Gentile et al. a .: Emerging biological insights and novel treatment strategies in multiple myeloma . In: Expert Opinion on Emerging Drugs . No. 17 (3) , September 2012, p. 407-438 , doi : 10.1517 / 14728214.2012.713345 .
- ^ A b P. Moreau: The Future of Therapy for Relapsed / Refractory Multiple Myeloma: Emerging Agents and Novel Treatment Strategies . In: Seminars in Hematology . No. 49 (1) , July 2012, p. 33-46 , doi : 10.1053 / j.seminhematol.2012.05.004 .
- ↑ a b Kerstin A. Gräfe: Panobinostat - New principle of action in myeloma. In: Pharmaceutical newspaper . 2015, accessed on December 28, 2015 (edition 39/2015).
- ↑ Vanessa Piechotta, Tina Jakob, Peter Langer, Ina Monsef, Christof Scheid: Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis . In: Cochrane Database of Systematic Reviews . November 25, 2019, doi : 10.1002 / 14651858.CD013487 ( wiley.com [accessed July 9, 2020]).
- ↑ Sheila A Fisher, Antony Cutler, Carolyn Doree, Susan J Brunskill, Simon J Stanworth: Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition . In: Cochrane Database of Systematic Reviews . January 30, 2019, doi : 10.1002 / 14651858.CD009768.pub2 ( wiley.com [accessed July 9, 2020]).
- ↑ Pomalidomide Celgene: EPAR - Summary for the public Summary of the European Public Assessment Report (EPAR), accessed on August 28, 2013.
- ↑ Pomalidomide Celgene: Summary of the EPAR for the public (PDF; 84 kB) EMA - European Medicines Agency (German), accessed on August 28, 2013.
- ↑ M. Dimopoulos et al .: Lenalidomide plus Dexamethasone for Relapsed or Refractory Multiple Myeloma . In: N Engl J Med . No. 357 , 2007, p. 2123-2132 ( abstract ).
- ^ V. Kotla et al .: Mechanism of action of lenalidomide in hematological malignancies . In: J Hematol Oncol . 12, No. 2, August 12, 2009, p. 36. doi : 10.1186 / 1756-8722-2-36 . PMID 19674465 . PMC 2736171 (free full text).
- ↑ European Commission Approves Kyprolis® (carfilzomib) For Combination Use In The Treatment Of Patients With Relapsed Multiple Myeloma , PM AMGEN of November 19, 2015, accessed on November 23, 2015
- ↑ EMA approves Ninlaro (PDF) PM EMA October 2017, accessed on July 3, 2018
- ↑ First histone deacetylase inhibitor approved in the EU. In: Pharmaceutical newspaper. September 14, 2015, accessed December 28, 2015 .
- ↑ HM Lokhorst et al .: Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma . In: The New England Journal of Medicine . 373, No. 13, September 24, 2015, pp. 1207-1219. doi : 10.1056 / NEJMoa1506348 . PMID 26308596 .
- ↑ adisinsight.springer.com
- ↑ Empliciti (elotuzumab) for injection, for intravenous use. Full prescribing information . Bristol-Myers Squibb Company. Archived from the original on December 8, 2015.
- ↑ Linus Knips, Nils Bergenthal, Fiona Streckmann, Ina Monsef, Thomas Elter: Aerobic physical exercise for adult patients with haematological malignancies . In: Cochrane Database of Systematic Reviews . January 31, 2019, doi : 10.1002 / 14651858.CD009075.pub3 ( wiley.com [accessed July 9, 2020]).
- ↑ a b S. Kumar et al .: International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma . In: Lancet Oncol . 17, No. 8, 2016, pp. E328 – e346. doi : 10.1016 / s1470-2045 (16) 30206-6 . PMID 27511158 .
- ^ FE Davies: Is molecular remission the goal of multiple myeloma therapy? . In: Hematology Am Soc Hematol Educ Program . 2017, No. 1, 2017, pp. 205–211. doi : 10.1182 / asheducation-2017.1.205 . PMID 29222257 .
- ↑ H. Ludwig et al .: Immunoglobulin heavy / light chain ratios improve paraprotein detection and monitoring, identify residual disease and correlate with survival in multiple myeloma patients . In: Leukemia . 27, No. 2, 2013, pp. 213-219. doi : 10.1038 / leu.2012.197 . PMID 22955329 .
- ↑ M. Michallet et al .: Heavy + light chain monitoring correlates with clinical outcome in multiple myeloma patients . In: Leukemia . 32, No. 2, 2017, pp. 376–382. doi : 10.1038 / leu.2017.209 . PMID 28663581 .
- ^ NC Munshi: Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis . In: JAMA Oncol . 3, No. 1, 2017, pp. 28–35. doi : 10.1001 / jamaoncol.2016.3160 . PMID 27632282 .
- ↑ (MRD) Molecular-genetic quantification of minimal residual disease - on the server of the Heidelberg University Hospital.