Pomalidomide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| 1: 1 mixture of ( R ) -isomer (top) and ( S ) -isomer | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Pomalidomide | |||||||||||||||||||||
| other names |
( RS ) -4-Amino-2- (2,6-dioxopiperidin-3-yl) -2,3-dihydro-1 H -isoindole-1,3-dione |
|||||||||||||||||||||
| Molecular formula | C 13 H 11 N 3 O 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Immunomodulator / antineoplastic |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 273.248 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.570 ± 0.06 g cm −3 |
|||||||||||||||||||||
| Melting point |
317.6-320.8 ° C |
|||||||||||||||||||||
| pK s value |
10.75 ± 0.40 |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Pomalidomide (trade name Pomalyst ; Imnovid ; manufacturer Celgene ) is a drug from the group of immunomodulators and is used in combination with low-dose dexamethasone in the treatment of multiple myeloma . It is used in patients who have stopped responding to lenalidomide and bortezomib . In studies, it led to a significant increase in the lifespan of the patients compared to the control groups. Pomalidomide is a thalidomide derivative.
Admission status
The first patent was granted in the USA in 1997 to the Celgene company . The patent number is US 5635517. Pomalidomide has been approved in the EU since August 2013 and is marketed under the trade name Imnovid.
synthesis
The synthesis of pomalidomide begins with the intramolecular formation of an imide from carbobenzyloxy-L-glutamine. The carboxylic acid group initially reacts with thionyl chloride to form the carboxylic acid chloride. This reaction is catalyzed by DMF . Then the ring closure takes place between the amide and the acid chloride . The carbobenzyloxy group acts as a protecting group during this sequence of reactions. Tetrahydrofuran is the solvent.
-
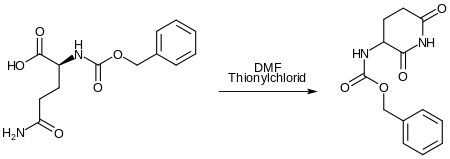
The protective group is split off by reductive hydrogenation. 3-aminopiperidine-2,6-dione is formed. -
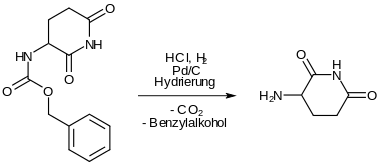
After adding 3-nitrophthalic anhydride, a nitro-substituted thalidomide analog is formed with heating. -

This is reduced to pomalidomide by reducing the nitro group. 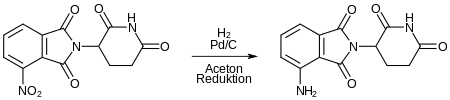
Analytics
Organic reactivity analysis
Quantitative reactivity analysis
Pomalidomide has a derivative of aniline as a structural element . The nitrogen of primary aromatic amines of the aniline derivatives can be determined quantitatively using the diazo titration method.
The glutarimide residue has a weakly NH-acidic bond, so that pomalidomide can be analyzed using argonto-acidimetric titration . The anion formed during the titration is precipitated as a sparingly soluble silver salt and the released protons are captured with pyridine. The resulting pyridinium ions can then be titrated as cationic acid with sodium hydroxide solution. A quantitative determination of the weak acid is also possible by anhydrous titration. Tetrabutylammonium hydroxide ( TBAH ) in dimethylformamide (DMF) can be used as a strong base for this.
Qualitative reactivity analysis
After diazotization, aniline derivatives can couple with N - (1-naphthyl) ethylenediamine (Bratton-Marshall reagent) or 2-naphthol .
The reaction with p -fluorobenzoyl chloride gives an N -benzoyl derivative with a characteristic melting point .
Primary aromatic amines react with Ehrlich's reagent to form Schiff's base .
Clinical information
Application areas (indications)
Pomalidomide in combination with dexamethasone is indicated for the treatment of relapsed and refractory multiple myeloma in adult patients who have received at least two previous therapies, including lenalidomide and bortezomib , and who have progressed on the previous therapy.
Contraindications (contraindications) / toxicology
Pomalidomide has been shown to be teratogenic in both rats and rabbits when used in the phase of essential organogenesis . Teratogenic effects in humans are to be expected if taken during pregnancy. Pomalidomide is absolutely contraindicated during pregnancy and breastfeeding. Women of childbearing potential should take care not to become pregnant while taking pomalidomide. This applies from four weeks before the planned start of therapy to four weeks after the end of therapy and also in the event of therapy interruptions. During this period women must commit to using two methods of contraception. However, not all methods of contraception are suitable. An estrogen - progestin combination is not recommended because of the increased risk of thrombosis . At the start of therapy, a weekly pregnancy test is done. This is followed by a monthly pregnancy check with a regular menstrual cycle. In the event of an irregular menstrual cycle, the check is carried out every two weeks. If you miss your period or if you have intermenstrual bleeding, a pregnancy test should be performed immediately and a doctor should be consulted. Should pregnancy occur despite all the precautionary measures, therapy must be stopped immediately and medical advice sought.
Adverse effects (side effects)
According to clinical studies, the most common adverse reactions include blood and lymphatic system disorders including anemia (45.7%), neutropenia (45.3%) and thrombocytopenia (27%), as well as general administration site symptoms including fatigue (28.3 %) %), Pyrexia (21%) and peripheral edema (13%). Infections and infestations, including pneumonia , are also common (10.7%). Peripheral neuropathies were found in 12.3% of the patients and venous embolic or thrombotic events (VTE) occurred in 3.3% of the patients. The most commonly reported Grade 3 or 4 adverse reactions include neutropenia (41.7%), anemia (27%), thrombocytopenia (20.7%), and infection or infestations including pneumonia (9%).
According to studies, side effects tend to occur more frequently in the first two courses of treatment with pomalidomide.
On April 29, 2015, the manufacturer announced in a Rote-Hand-Brief that the use of pomalidomide can lead to severe liver damage, interstitial lung diseases and heart failure.
Pharmacological properties
Mechanism of action (pharmacodynamics)
Pomalidomide works through a variety of mechanisms. The inhibition of the cell cycle means that degenerated cells cannot proliferate any further. This is done by activating p21 WAF-1 . In addition, caspase 8 activation and regulation of tumor suppressor and oncogenes lead to apoptosis of the myeloma cells. In addition, angiogenesis is inhibited. As part of the immunomodulatory effect of pomalidomide, T cells are co - stimulated and NK cells are stimulated. The formation of pro- inflammatory cytokines such as TNF-α and IL-6 is inhibited. In addition, other effects of pomalidomide are suspected, but these have not yet been conclusively researched.
Absorption and distribution in the body (pharmacokinetics)
Pomalidomide is quickly and well absorbed orally.
| Cmax | Tmax | Half-life | Plasma protein binding | Volume of Distribution | Urine clearance | AUC 0 |
|---|---|---|---|---|---|---|
| 13 ng / mL (2 mg, oral) | 3 h | 6.5-8 h | 12-44% | 62-138 L. | renal 73% | 189 ng.h / mL |
The metabolism of pomalidomide takes place predominantly via hydrolysis of the glutarimide ring, hydroxylation (CYP3A4 and CYP1A2) of the phthalimide ring in the 5-position and subsequent glucuronidation. The main metabolite is 5-hydroxy-glucuronide pomalidomide. 73% of the excretion is renal and 15% faecal.
Experiments have shown that the enantiomerically pure form of pomalidomide results in rapid isomerization in humans , so that a racemic mixture with equal parts of the ( R ) and ( S ) form is present in the body.
Individual evidence
- ↑ a b c SciFinder. Retrieved July 6, 2014 .
- ↑ a b Patent: WO2013 / 126326 A1, CELGENE CORPORATION; COHEN, Benjamin, M .; LI, Ying; XU, Jean; LEONG, William, W .; MAN, Hon-wah; 2013.
- ↑ Product information Pomalidomide ( Memento from June 3, 2015 in the Internet Archive ) at selleckchem.com, accessed on July 16, 2014.
- ↑ a b Data sheet Pomalidomide, ≥98% (HPLC) from Sigma-Aldrich , accessed on September 25, 2014 ( PDF ).
- ↑ Myelodysplastic Syndromes (MDS) and Multiple Myeloma (MM). In: Viennese clinical magazine. 16, 2013, pp. 30-31, doi: 10.1007 / s00740-013-0176-6 .
- ↑ a b Pomalidomide (Imnovid ®) newly approved for multiple myeloma . (PDF) In: Onkologie (International Journal for Cancer Research and Treatment), Vol. 35, No. October 10th, 2013; Retrieved July 5, 2014.
- ↑ Nicola Siegmund-Schultze: Multiple Myeloma: Pomalidomide is a new option for relapse . In: Deutsches Ärzteblatt . tape 110 , no. 50 , December 13, 2013, p. A-2428 / B-2139 / C-2061 .
- ↑ GW Muller, R. Chen, SY Huang, LG Corral, LM Wong, RT Patterson, Y. Chen, G. Kaplan, DI Stirling: Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production. In: Bioorganic & medicinal chemistry letters. Volume 9, Number 11, June 1999, pp. 1625-1630, PMID 10386948 .
- ↑ Eberhard Ehlers: Analytics II. Short textbook quantitative and instrumental analytics . Stuttgart 2008, pp. 218, 223-224.
- ↑ Eberhard Ehlers: Analytics II. Short textbook quantitative and instrumental analytics . Stuttgart 2008, pp. 218, 123-124.
- ↑ Eberhard Ehlers: Analytics II. Short textbook quantitative and instrumental analytics . Stuttgart 2008, pp. 218, 141-142.
- ^ A. Calvin Bratton, EK Marshall, Jr. and with the technical assistance of Dorothea Babbitt and Alma R. Hendrickson: A new coupling component of sulfanilamide determination . In: The Journal of Biological Chemistry . 128, May 1939, pp. 537-550.
- ↑ Michael J. Robarge, Roger Shen-Chu Chen, George W. Muller, Hon-Wah Man: Isoindole-imide compounds, compositions, and uses thereof . Patent: US2003 / 45552 A1, 2003.
- ↑ NM Ibrahim, KJ Hamad, SH Al-Joroshi: Synthesis and characterization of some ship bases . In: Asian Journal of Chemistry . 18, No. 3, 2006, pp. 2404-2406.
- ↑ Imnovid specialist information . November 2015.
- ↑ a b Rote-Hand-Brief on Imnovid. (PDF) Retrieved July 6, 2014 .
- ^ Medication Guide Pomalyst® . (PDF) fda.gov. Retrieved July 24, 2014.
- ↑ Pomalidomide (Imnovid®): New important safety information to minimize the risk of serious hepatotoxicity, interstitial lung disease and heart failure. (PDF) Celgene GmbH, April 29, 2015, accessed April 30, 2015 .
- ↑ Assessment report Pomalidomide Celgene (PDF) European Medicines Agency.
- ↑ Hoffmann M, Kasserra C, Reyes J, Schafer P, Kosek J, Capone L, Parton A, Kim-Kang H, Surapaneni S, Kumar G .: Absorption, metabolism and excretion of [14C] pomalidomide in humans following oral administration . In: Cancer Chemotherapy and Pharmacology . 72, No. 2, February 2013, pp. 489-591. doi : 10.1007 / s00280-012-2040-6 . PMID 23203815 .
- ↑ Yan Li, Simon Zhou, Matthew Hoffmann, Gondi Kumar, Maria Palmisano: Modeling and Simulation to Probe the Pharmacokinetic Disposition of Pomalidomide R- and S-Enantiomers . In: Journal of Pharmacology and Experimental Therapeutics . 350, pp. 265-272. doi : 10.1124 / jpet.114.215251 . PMID 24833703 .


