Monoclonal gammopathy
| Classification according to ICD-10 | |
|---|---|
| D47.2 | Monoclonal gammopathy |
| ICD-10 online (WHO version 2019) | |
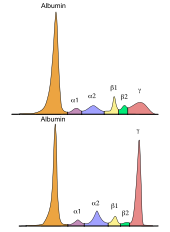
When monoclonal gammopathy is a collective term of various malignant as well as non-malignant (pre) diseases associated with a monoclonal proliferation of plasma cells and an increased production of a single immunoglobulin (antibodies, IgG , IgA , IgM , IgD or IgE ) or a fragment thereof (e.g. free light chains or heavy chains) accompanied. In most cases, both intact antibodies and free light chains are produced, but only intact antibodies or only free light chains can occur. These products ( monoclonal antibodies ) are also known as M-protein. In rare cases, neither intact antibodies nor free light chains are produced by the plasma cells. In the ideal case, the M protein can be detected in the so-called γ fraction by electrophoretic methods (e.g. in serum protein electrophoresis ).
In addition to the known symptomatic forms the multiple myeloma (MM) and the plasmacytoma and its asymptomatic precursors Smoldering multiple myeloma (SMM), or monoclonal gammopathy of undetermined significance (MGUS) are Waldenstrom , the AL amyloidosis , the Monoclonal gammopathy renal significance ( MGRS) as well as various less common clinical pictures of monoclonal gammopathies. Above all, the monoclonal gammopathies belong to the indolent (low-grade malignant) B-cell non-Hodgkin lymphomas , the symptomatic forms being malignant cancers .
distribution
| Frequency of various forms of monoclonal gammopathies |
||
|---|---|---|
 |
||
| Type | frequency | |
| MGUS | 51% | |
| SMM | 6% | |
| Multiple myeloma | 18% | |
| AL amyloidosis | 11% | |
| Lymphoproliferative | 4% | |
| Waldenström's disease | 3% | |
| Solitary plasmacytoma | 1 % | |
| Other | 6% | |
Due to the different forms of monoclonal gammopathies, there is no general prevalence or incidence . For example, the prevalence of MGUS in people aged 45 to 75 years is 3.5%. In Germany, the incidence of multiple myeloma is 4–5 new cases per 100,000 population and year. In general, MGUS, AL amyloidosis, and multiple myeloma are the two most common forms of monoclonal gammopathy.
root cause
Changes in hereditary information are particularly possible causes for the development of monoclonal gammopathies . Mutations of various genes and translocations have been described in the literature. These changes are favored by various factors. An influence of obesity , radioactive radiation , autoimmune diseases , inflammatory processes and infections , pesticides and the presence of certain single nucleotide polymorphisms have been described for the development of monoclonal gammopathy of unclear significance .
Pathogenesis
A chromosomal translocation of genes which code for the heavy chains of immunoglobulins is typical in the development of monoclonal gammopathies . A negative prognosis can be made especially for the translocation t (4; 14), t (14; 16) and t (14; 20). In addition, hyperdiploidies and deletions have also been described as changes that can be the cause of the pathogenesis. There is a connection here for the deletions 1p, 13q and 17p. There are similar data for monosomy 13 and duplication 1q. In the further course, mutations can occur that can cause the progression of monoclonal gammopathy and thus the development of a symptomatic disease. For example, mutations in the N- and K- Ras , Myc and p53 genes are known . Even changes that the NF-kB - pathway activated can contribute to the development of monoclonal gammopathy. Mutations in the genes MYD88 , CXCR4 and ARID1, among others , have been identified specifically for the development of Waldenström's disease .
Clinical manifestations
Monoclonal gammopathies are generally characterized by typical, albeit unspecific, symptoms. In principle, a distinction is made between the asymptomatic precursors MGUS and SMM, as well as the various symptomatic forms such as multiple myeloma, Waldenström's disease and AL amyloidosis. For example, patients who suffer from a symptomatic form often complain of bone pain, fatigue and weakness. An adequate examination can detect hypercalcaemia , anemia , abnormal accumulation of clonal plasma cells in the bone marrow , osteolysis , deposits of monoclonal proteins in various tissues, impaired kidney function or proteinuria . Symptoms may or may not appear at the same time. More and more recent studies have shown the occurrence of symptoms in the preliminary stages MGUS and SMM, some of which can also be associated with serious consequences. So in the meantime z. For example, a purely asymptomatic MGUS is differentiated from a symptomatic MGRS, which at the same time does not meet the criteria of multiple myeloma. The clinical appearance of various monoclonal gammopathies can be quite different, which is why the division into asymptomatic and symptomatic forms is of particular interest with regard to therapy. A brief overview can be found in the Examination Methods section of this article.
Investigation methods
General
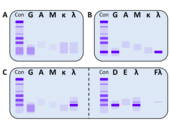
Monoclonal gammopathy is often diagnosed by chance, especially in the early stages. The suspicion often arises from an abnormal serum protein electrophoresis with a typical band in the gel or peak in the histogram (so-called M-gradient), which can be found in many (but not all) cases in the γ-fraction. For an unambiguous determination and characterization of the M protein, an additional immunofixation and the determination of the free light chains in the serum are necessary. Pathological concentrations of free light chains can sometimes also be detected in urine. One then speaks of a Bence Jones proteinuria . In many cases, the symptoms can also be an indication of the presence of monoclonal gammopathy. Certain patient groups (e.g. osteoporosis and polyneuropathy patients) also show an increased prevalence for the presence of monoclonal gammopathy, which should be taken into account during the examination.
Exclusion diagnostics
A comprehensive diagnosis of exclusion can include the following examination methods, which can be expanded if necessary:

| Overview of exclusion diagnostics | ||
|---|---|---|
| Laboratory diagnostics | histology | Imaging methods |
|
||
differentiation
The diagnostic criteria and the typical clinical course of various monoclonal gammopathies are given below. Some of these forms can be further subdivided and also appear combined. For example, monoclonal gammopathies in which the kidney is involved can be classified as MGRS. Typical for the classification of a symptomatic disease, in particular multiple myeloma, is the presence of a CRAB or SLiM criterion ( CRAB criteria , from the English for Hyper C alcemia ( hypercalcemia ), R enal Insufficiency ( renal insufficiency ), A nemia ( anemia ) and B one Lesions ( osteolyses ); SLiM criteria also from English for S ixty percent bone marrow plasma cells (≥ 60% clonal plasma cells in the bone marrow), Involved: uninvolved serum free Li ght chain ratio ≥100 (ratio of the involved to non-involved free light chain in serum ≥ 100; the concentration must be ≥ 100 mg / l) and > 1 focal lesions on M RI studies (> 1 bone lesion, detected by MRI)). There are also other specific criteria for making a more precise differentiation. The most prominent examples are:
| Diagnostic criteria and clinical course of monoclonal plasma cell diseases (selection) | |
|---|---|
| illness | Diagnostic criteria |
| MGUS |
|
| SMM |
|
| MM |
|
| Waldenström's disease | |
| AL amyloidosis |
|
treatment
In symptomatic diseases, in which an improvement is only possible through the targeted suppression of the underlying plasma cell clone, the use of chemotherapy , stem cell transplantation and the administration of modern pharmaceuticals such as monoclonal therapeutic antibodies are the methods of choice. For some time now, therapeutic monoclonals have been particularly effective Antibodies are available and have already shown promising results in clinical studies. In addition to these curative therapeutic methods , palliative therapeutic agents can help improve the general condition of the patient. A decision on the therapy to be carried out must be made on the basis of all available findings individually and depending on the present form of monoclonal gammopathy.
prevention
Various risk factors such as obesity, radiation and harmful chemicals like pesticides should be avoided as these are discussed as triggers of the disease. According to the current state of knowledge, further preventive measures for the primary development of monoclonal gammopathy cannot be taken. A regular examination of already ill patients can, however, prevent further development and worsening of the disease, its symptoms and many accompanying symptoms.
Prospect of healing
The healing prospects depend on the underlying monoclonal gammopathy and cannot be generalized here. In the case of malignant diseases, the progressive development of highly efficient therapeutics has brought a cure within reach. As with all cancers, there is always a residual risk of recurrence . Regular examination of the patient can identify a relapse at an early stage and a therapy decision can be made immediately.
Web links
- onkopedia.de
- Myeloma Germany eV
- Competence network malignant lymphomas
- wikilite.com
- International Myeloma Foundation
- International Myeloma Working Group
Individual evidence
- ^ GP Mead et al .: Serum free light chains for monitoring multiple myeloma . In: Br J Hematol . 126, No. 3, March 29, 2004, pp. 348-354. doi : 10.1111 / j.1365-2141.2004.05045.x . PMID 15257706 .
- ^ The International Myeloma Working Group: Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group . In: Br J Hematol . 121, No. 5, September 2, 2002, pp. 749-757. PMID 12780789 .
- ^ N. Leung, SV Rajkumar,: Renal Manifestations of Plasma Cell Disorders . In: American Journal of Kidney Diseases . No. 50 , 2007, p. 155-165 ( abstract ).
- ↑ a b c N Leung et al .: Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant . In: Blood . 120, No. 22, October 9, 2012, pp. 4292-4295. doi : 10.1182 / blood-2012-07-445304 . PMID 23047823 .
- ↑ a b RA. Kyle et al .: Monoclonal gammopathy of undetermined significance . In: Br J Haematol . 134, No. 6, 2006, pp. 573-589. doi : 10.1111 / j.1365-2141 . PMID 16938117 .
- ↑ RA. Kyle et al .: Prevalence of monoclonal gammopathy of undetermined significance . In: N Engl J Med . 354, No. 13, 2006, pp. 1362-1369. doi : 10.1056 / NEJMoa054494 . PMID 16571879 .
- ^ Society of Epidemiological Cancer Registers in Germany eV (GEKID) extrapolation from the Institute for Cancer Epidemiology eV, Lübeck for icd10: C90 based on the data from the cancer registers BY, BR, HB, HH, MV, NI, NW (Reg.Bez. Münster) SL , SN, SH (2005-2009), Retrieved October 27, 2017.
- ↑ a b c d e f g h i j N van de Donk et al .: The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network . In: Haematologica . 99, No. 6, March 21, 2014, pp. 984-96. doi : 10.3324 / haematol.2013.100552 . PMID 23224402 . PMC 4040895 (free full text).
- ^ GJ Morgan et al .: The genetic architecture of multiple myeloma . In: Nat Rev Cancer . 12, No. 5, May 2012, pp. 335-48. doi : 10.1038 / nrc3257 . PMID 22495321 .
- ↑ a b M Chesi et al .: Advances in the pathogenesis and diagnosis of multiple myeloma . In: Int Jnl Lab Hem . 2015, pp. 108–114. doi : 10.1111 / ijlh.12360 . PMID 25976968 .
- ^ R Fonseca et al .: International Myeloma Working Group molecular classification of multiple myeloma: spotlight review . In: Leukemia . 23, No. 12, December 2009, pp. 2210-21. doi : 10.1038 / leu.2009.174 . PMID 19798094 . PMC 2964268 (free full text).
- ^ NC Munshi et al .: Guidelines for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2 . In: Blood . 117, No. 18, May 2011, pp. 4696-4700. doi : 10.1182 / blood-2010-10-300970 . PMID 21292777 . PMC 3293763 (free full text).
- ^ A Zingone et al .: Pathogenesis of Monoclonal Gammopathy of Undetermined Significance (MGUS) and Progression to Multiple Myeloma . In: Semin Hematol . 48, No. 1, January 1, 2012, pp. 4–12. doi : 10.1053 / j.seminhematol.2010.11.003 . PMID 21232653 . PMC 3040450 (free full text).
- ^ A b c SV Rajkumar et al .: International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma . In: Lancet . 15, No. 12, November 15, 2014, pp. E538-48. doi : 10.1016 / s1470-2045 (14) 70442-5 . PMID 25439696 .
- ↑ A Dispenzieri et al .: International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders . In: Leukemia . 23, No. 2, February 2009, pp. 215-224. doi : 10.1038 / leu.2008.307 . PMID 19020545 .
- ↑ T Golombick et al .: Prevalence of monoclonal gammopathy of undetermined significance / myeloma in patients with acute osteoporotic vertebral fractures . In: Acta Haematol . 120, No. 2, October 14, 2008, pp. 87-90. doi : 10.1159 / 000162282 . PMID 18852483 .
- ^ N Steiner et al .: Are neurological complications of monoclonal gammopathy of undetermined significance underestimated? . In: Oncotarget . 8, No. 3, December 10, 2016, pp. 5081-5091. doi : 10.18632 / oncotarget.13861 . PMID 27974705 . PMC 5354894 (free full text).
- ↑ SM Ansell et al .: Diagnosis and management of Waldenstrom macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines . In: Mayo Clin Proc . 85, No. 9, September 2010, pp. 824-833. doi : 10.4065 / mcp.2010.0304 . PMID 20702770 . PMC 2931618 (free full text).
- ^ RG Owen et al .: Guidelines on the diagnosis and management of Waldenstrom macroglobulinaemia . In: Br J Hematol . 165, No. 3, May 2014, pp. 316–333. doi : 10.1111 / bjh.12760 . PMID 24528152 .
- ^ J Gillmore et al .: Guidelines on the diagnosis and investigation of AL amyloidosis . In: Br J Hematol . 168, No. 2, October 14, 2014, pp. 207-18. doi : 10.1111 / bjh.13156 . PMID 25312307 .
- ↑ SH Nasr et al .: Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution . In: Clin J Am Soc Nephrol . 7, No. 2, December 14, 2011, pp. 231-9. doi : 10.2215 / CJN.08640811 . PMID 23047823 .
- ^ Mateos & González-Calle: Smoldering Multiple Myeloma: Who and When to Treat . In: Clin Lymphoma Myeloma Leuk . 17, No. 11, November 2017, pp. 716–722. doi : 10.1016 / j.clml.2017.06.022 . PMID 28709797 .
- ↑ a b MA Bärtsch et al .: Current aspects in the diagnosis and therapy of plasma cell myeloma . In: Dtsch Med Wochenschr . 142, No. 11, 2017, pp. 800-804. doi : 10.1055 / s-0043-100295 .
- ↑ JP Bridoux et al .: How I treat monoclonal gammopathy of renal significance (MGRS) . In: Blood . 122, No. 22, October 9, 2013, pp. 3583-3590. doi : 10.1182 / blood-2013-05-495929 . PMID 24108460 .
- ↑ MA Dimopoulos et al .: Treatment recommendations for patients with Waldenstrom macroglobulinemia (WM) and related disorders: IWWM-7 consensus . In: Blood . 124, No. 9, July 17, 2014, pp. 1404-11. doi : 10.1182 / blood-2014-03-565135 . PMID 4148763 .
- ↑ MA Gertz et al .: Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment . In: Am J Hematol . 91, No. 9, August 17, 2016, pp. 947-56. doi : 10.1002 / ajh.24433 . PMID 27527836 .