Gadoteric acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Gadoteric acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 16 H 25 GdN 4 O 8 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class |
Paramagnetic contrast agent |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 558.7 g mol −1 as Gd-DOTA | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
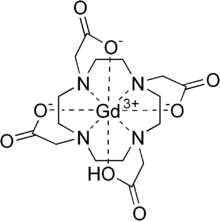
Gadoteric acid , often also referred to as Gd-DOTA , is the international non-proprietary name for a contrast agent that is used in magnetic resonance imaging (MRI).
Structure and working principle of the contrast agent
One molecule of gadoteric acid contains a gadolinium ion which, with the help of the strong complexing agent 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), is present in complexed form. Complexation is very important because free, uncomplexed gadolinium ions are toxic to human and most animal organisms. The complex formation constant of DOTA is above 10 20 at a pH value of 7 . Gadoteric acid is the most stable approved gadolinium complex with the longest dissociation half-life.
Gadolinium has seven unpaired electrons on the outer electron shell (f-shell) , which give the element a strong paramagnetism . Protons , such as those found in the water of body fluids, relax much more quickly in the vicinity of gadolinium. In particular, the so-called T 1 time is considerably shortened by the gadolinium. Areas in which the contrast agent is concentrated are therefore shown brighter in T 1 -weighted images than other structures. This significantly improves the image quality of an MRI image and increases the contrast between pathologies and the surrounding normal tissue.
Gadoteric acid is a non-specific contrast medium that accumulates in all organs outside the central nervous system . In the initial perfusion phase, the gadoteric acid spreads in the intravascular space and then quickly enters the extracellular space . The glomerular filtration rate (GFR) in accordance with it is the kidneys, that is on the kidney unchanged (no metabolism , no dissociation and no retention ) excreted. Because Gd-DOTA is non-selectively distributed in the extracellular space of all extra-cerebral tissues, it cannot be used organ-specifically. Because of its hydrophilicity , Gd-DOTA cannot pass through intact cell membranes . Therefore, after intravenous administration, it can only be found in the intravascular space and in the interstitium . Because of the very low protein binding , it is excreted relatively quickly via the kidneys.
Gadoteric acid was first approved in some European countries in 1989 as a second MRI contrast agent (after gadopentetate dimeglumine (Gd-DTPA)) . As a highly polar and relatively large molecule, gadoteric acid is unable to cross the blood-brain barrier in a healthy person . In some diseases, such as glioblastoma , however, it can cross the damaged blood-brain barrier and penetrate the diseased tissue. This makes it possible to obtain more precise information about the type and location of the tumor. In the imaging, the tumor is also better delineated from the healthy tissue. Overcoming the blood-brain barrier is an important diagnostic criterion for brain tumors.
In the human organism, the distribution half-life is about 2 to 3 minutes, while the plasma half-life is about 90 minutes. The mean hydrodynamic diameter is approximately 5 nm. The molecular diameter is approximately 0.9 nm.
Side effects
Gadoteric acid is very well tolerated. Occasionally (in 0.1 - 1 percent of those treated) hypersensitivity reactions occur. Allergic reactions are more common in patients with a known intolerance to contrast media (including those containing iodine), asthma under treatment or patients with multiple allergies. As with all gadolinium-containing contrast media, nausea, vomiting, rashes, tremors, dyspnoea , bronchospasm , headache, metallic taste or even heat when injecting can occur. In the case of higher doses and pre-existing renal insufficiency or kidney disease, contrast medium-induced acute renal insufficiency may occur in rare cases. This usually occurs after 48 to 72 hours and is mostly reversible . Because of the high osmolality of the solution of 1300 mOsm / kg water and the associated risk of high local concentrations, unintentional extravascular injection must be avoided at all costs . In contrast to other gadolinium-based contrast media, no nephrogenic systemic fibrosis caused by gadoteric acid has been observed in any of the more than 25 million patients treated .
Megluminate
In solution, one mole of gadoteric acid is mixed with one mole of meglumine ( N -methyl- D -glucamine). In these cases, one speaks of the meglumine salt of gadoteric acid , gadoterate meglumine , or hydrogen- [1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetato (4)] gadolinium meglumine.
Trade names
Artirem (D, A, CH) with a gadolinium concentration of 0.0025 mmol / ml in the form of gadoterate meglumine has been approved for contrast-enhanced direct MR arthrography since 2002 ( Guerbet ). The iso-osmolar solution is injected directly into the joint space (intra-articular).
Dotarem (D, A, CH) with a gadolinium concentration of 0.5 mmol / ml (Guerbet) is approved for contrast agent imaging of CNS lesions, for whole-body MR contrast agent examinations and contrast agent- assisted MR angiography . The usual dosage is 0.1 mmol / kg body weight, corresponding to 0.2 ml / kg body weight intravenously. A maximum of 0.3 mmol / kg body weight can be administered to patients with healthy kidneys, but such a high dose is rarely indicated.
Dotagita (D), Cyclolux (D) and Clariscan are generic drugs with similar approval and the same dosage as Dotarem .
literature
- P. Reimer, R. Vosshenrich: Contrast media in the MRT. In: Der Radiologe , 44/2004, pp. 273-83.
Web links
- L. Wachsmuth: Introduction to the basics of medical physics (PDF file; 5.25 MB)
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ CU Herborn et al .: Clinical safety and diagnostic value of the gadolinium chelate gadoterate meglumine (Gd-DOTA). In: Invest Radiol 42/2007, pp. 58-62.
- ↑ P. Caravan et al .: Gadolinium (III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. In: Chemical Reviews 99/1999, pp. 2293-352.
- ↑ a b C. Reinländer: MRI contrast media for the bone marrow: Comparative experimental studies of USPIO, SPIO and Gd-DOTA. Dissertation, Westfälische Wilhelms-Universität Münster, 2003.
- ↑ N. Kaufels: MRI myocardial examinations for vitality and perfusion with P792 in comparison with Gd-DOTA in pigs after induction of an acute myocardial infarction. Dissertation, FU Berlin, 2006.
- ^ HHJ Hager, F. von Bruchhausen: Hager's handbook of pharmaceutical practice. Springer, ISBN 3-540-62644-1 .
- ↑ PR Newswire Europe Ltd. Contrast agents for magnetic resonance imaging and nephrogenic systemic fibrosis in severe renal insufficiency , accessed June 2, 2008.
- ↑ KJ Murphy et al .: Adverse reactions to gadolinium contrast media: a review of 36 cases. In American Journal of Roentgenology 167/1996, pp. 847-9.
- ↑ HS Thomsen et al .: Is there a causal relation between the administration of gadolinium-based contrast media and the development of nephrogenic systemic fibrosis (NSF)? In: Clinical Radiology 61/2006, pp. 905-6.
- ^ HHJ Hager, F. von Bruchhausen: Hager's handbook of pharmaceutical practice.