Hydrates
Hydrates ( ancient Greek ὕδωρ hydōr 'water') are generally substances in chemistry that contain water . The term is treated somewhat differently in different areas of chemistry. In contrast to hydrates, there are anhydrides , compounds from which water has been removed.
Inorganic hydrates
Inorganic hydrates are solvates , so they contain molecular water, for example as crystal water . In addition to crystals containing water of crystallization, complexes that have water ligands are also referred to as hydrates. Substances without water of crystallization are also called Called anhydrates .
The water of crystallization in hydrates can be bound to certain ions by hydrogen bonds or other intermolecular forces, but it can also be the case that it is mainly incorporated because this creates a more favorable crystal structure . In crystalline hydrates there are certain stoichiometric ratios between ionic compounds and crystal water. In many cases, several hydrates with different amounts of water of crystallization are also possible. Modifications of sodium carbonate with one, two, five, seven and ten equivalents of water of crystallization and sodium carbonate without water of crystallization are known.
Hydrates arise through hydration , mostly during crystallization from aqueous solutions. Hydrates rich in water of crystallization can be dehydrated, for example by heating, so that either other hydrates containing less water of crystallization are formed or the anhydrous compound, with color changes often occurring at the same time, e.g. B. for cobalt (II) chloride:
For the identification of hydrates in empirical formulas , this is not inserted directly into the formula, but is placed at the end of the empirical formula as n H 2 O (n is the amount of water of crystallization that occurs per formula unit in the crystal). CaSO 4 · 2 H 2 O describes, for example, calcium sulfate with two equivalents of crystal water, which corresponds to gypsum . A -xxxhydrate is added to the name of the compound , where xxx is a Greek numeral for the equivalent number of crystal water. Accordingly, gypsum is systematically called calcium sulfate dihydrate.
| Surname | Number of water molecules |
Examples ( salts ) | chem. formula |
|---|---|---|---|
| At hydrate | 0 | Magnesium chloride anhydrate | MgCl 2 |
| Hemi hydrate | ½ | Calcium sulfate hemihydrate | CaSO 4 • 0.5H 2 O |
| mono hydrate | 1 |
Sodium hydrogen sulfate monohydrate
Cesium thiosulfate monohydrate |
NaHSO 4 · H 2 O
Cs 2 [S 2 O 3 ] · H 2 O |
| Sesqui hydrate | 1.5 | Potassium carbonate sesquihydrate | K 2 CO 3 • 1.5 H 2 O |
| Di hydrate | 2 |
Calcium sulfate dihydrate, calcium chloride dihydrate |
CaSO 4 · 2H 2 O CaCl 2 · 2H 2 O |
| Tri hydrate | 3 |
Sodium acetate trihydrate, lead (II) acetate trihydrate |
NaC 2 H 3 O 2 • 3 H 2 O PbC 4 H 6 O 4 • 3 H 2 O |
| Tetra hydrate | 4th | Potassium Sodium Tartrate Tetrahydrate | KNaC 4 H 4 O 6 · 4 H 2 O |
| Penta hydrate | 5 | Copper sulfate pentahydrate | CuSO 4 · 5 H 2 O |
| Hexa hydrate | 6th |
Aluminum chloride hexahydrate, cobalt (II) chloride hexahydrate |
AlCl 3 · 6H 2 O CoCl 2 · 6H 2 O |
| Hepta hydrate | 7th |
Magnesium sulfate heptahydrate, iron (II) sulfate heptahydrate, zinc (II) sulfate heptahydrate |
MgSO 4 • 7 H 2 O FeSO 4 • 7 H 2 O ZnSO 4 • 7 H 2 O |
| Octa hydrate | 8th | Praseodymium (III) sulfate octahydrate | Pr 2 (SO 4 ) 3 · 8H 2 O |
| Nona hydrate | 9 | Chromium (III) nitrate nonahydrate | Cr (NO 3 ) 3 · 9H 2 O |
| Deca hydrate | 10 |
Sodium sulfate decahydrate (Glauber's salt), sodium carbonate decahydrate |
Na 2 SO 4 · 10 H 2 O Na 2 CO 3 · 10 H 2 O |
| Undeca hydrate | 11 | Magnesium chromate undecahydrate | Mg [CrO 4 ] · 11H 2 O |
| Dodeca hydrate | 12 | Sodium phosphate dodecahydrate | Na 3 PO 4 • 12 H 2 O |
Organic hydrates
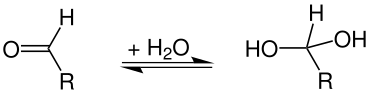
In organic chemistry, hydrated compounds are called hydrates. These are mostly not compounds that contain molecular water, but those in which water has been chemically added to a compound through an addition reaction . Examples are geminal diols or aldehyde hydrates , which are hydrated aldehydes :
Most aldehyde hydrates are unstable, with the exception of chloral hydrate and formalin . Geminal triols (orthocarboxylic acids = hydrated carboxylic acids ), like orthocarbonic acid (tetrahydroxymethane), are only stable in the form of their esters .
There are also stable organic compounds that contain water of hydration, for example ( R ) - cysteine · hydrochloride · monohydrate [ L- cysteine · hydrochloride · monohydrate], which is produced on an industrial scale.
Gas hydrates
In the case of gas hydrates , in contrast to substances containing water of crystallization, it is not the water in the substance but an otherwise gaseous compound such as methane , hydrogen sulfide or carbon dioxide that is enclosed in ice . A clathrate is formed , an inclusion compound without any great physical attraction forces between ice and gas. The best known gas hydrate is methane hydrate .
carbohydrates
Carbohydrates contain -hydrate in their name, but do not belong to the hydrate group.
literature
- Entry to hydrates. In: Römpp Online . Georg Thieme Verlag, accessed on June 3, 2012.