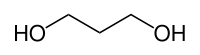
1,3-propanediol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 1,3-propanediol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 3 H 8 O 2 | |||||||||||||||||||||
| Brief description |
colorless and odorless liquid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 76.10 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.05 g cm −3 (20 ° C) |
|||||||||||||||||||||
| Melting point |
−26 ° C |
|||||||||||||||||||||
| boiling point |
213 ° C |
|||||||||||||||||||||
| Vapor pressure |
|
|||||||||||||||||||||
| solubility |
completely miscible with water |
|||||||||||||||||||||
| Refractive index |
1.4383 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−480.8 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
1,3-Propanediol (PDO) is a chemical compound . It consists of the basic structure of propane with a hydroxyl group at each terminal position . 1,3-Propanediol belongs to the group of dihydric alcohols , the diols .
Extraction and presentation
1,3-Propanediol is synthetically accessible in a number of ways . One possibility is the hydration of acrolein with dilute sulfuric acid in the presence of hydroquinone (prevents the polymerisation of the acrolein) and subsequent hydrogenation with Raney nickel as a catalyst . It can also be produced from 3-hydroxypropionic acid .
A biochemical synthesis of 1,3-propanediol from renewable raw materials is also possible. Naturally, it can be formed from the fermentation of glycerin by many anaerobic bacteria. Possible genera are Citrobacter , Clostridium , Enterobacter , Klebsiella and Lactobacillus . An Escherichia coli strain is used industrially, which can ferment glucose to 1,3-propanediol through strong genetic changes . Corn is used as a raw material . In 2013, almost half of total production was due to this type of production.
properties
Physical Properties
It is a colorless, hygroscopic liquid with only a slight odor that boils at 213 ° C. It is miscible with water, alcohols, ethers and formamide, and is sparingly soluble in benzene and chloroform.
The vapor pressure function results according to Antoine according to log 10 (P) = A− (B / (T + C)) (P in kPa, T in K) with A = 8.34759, B = 3149.87 and C = 9, 1444. The compound is difficult to ignite. Above its flash point, it forms inflammable vapor-air mixtures. The flash point is 128 ° C. The lower explosion limit is around 2.5 vol.% (79 g · m −3 ). The ignition temperature is 400 ° C. The substance therefore falls into temperature class T2.
Chemical properties
1,3-Propanediol undergoes many typical reactions of alcohols, such as etherifications and esterifications . Both hydroxyl groups are equally reactive. 1,3-Propanediol can also undergo polycondensation as a diol . One example is the formation of the polyester polytrimethylene terephthalate (PPT) with terephthalic acid :
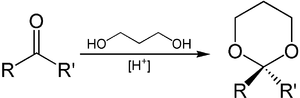
1,3-Propanediol can be used to introduce protective groups on aldehydes and ketones . A derivative of 1,3-dioxane , an acetal or ketal , is formed from the carbonyl compound used under acidic conditions . This is stable against basic conditions and, if protection is no longer required, can be split again by the action of Brønsted or Lewis acids in the presence of water.
The synthesis of 1,3-dioxane itself is also possible by reacting 1,3-propanediol with formaldehyde and phosphoric acid . This reaction can also be carried out using hydrochloric acid and urotropine .
use
1,3-Propanediol is mainly used to manufacture the polyester polytrimethylene terephthalate (PTT). There are also numerous other applications in polymer chemistry: It is used for the production of polycarbonate and polymerization catalysts and as a crosslinking agent for polyacrylates and polymethacrylates. It is also the starting material for compounds that are used in the production of polyurethanes , epoxy resins and rubber . Further applications in polymer chemistry, such as B. as a component in coatings or as a bio-based alternative to other diols .
Apart from polymer chemistry, 1,3-propanediol can also be used as an antifreeze and coolant. In addition, it is used for the production of UV-stable lacquers in which the relationship between elasticity and hardness is balanced and is contained in cosmetics, printing inks and coating materials.
Individual evidence
- ↑ Entry on PROPANEDIOL in the CosIng database of the EU Commission, accessed on February 25, 2020.
- ↑ a b Entry on propanediol. In: Römpp Online . Georg Thieme Verlag, accessed on September 29, 2014.
- ↑ a b c d e f g h i j k l Entry for CAS no. 504-63-2 in the GESTIS substance database of the IFA , accessed on April 3, 2018(JavaScript required) .
- ↑ M. Bergmann, A. Miekeley, E. v. Lippmann: On the chemistry of associating lactolides: On transformations of aldols. In: Chem. Ber. 62, 1929, pp. 1467-1474. doi: 10.1002 / cber.19290620616 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-25.
- ^ CH Werkman, GF Gillen: Bacteria Producing Trimethylene Glycol. In: J. Bacteriol. 23, 1932, pp. 167-182.
- ↑ Cristina Della Pina, Ermelinda Falletta, Michele Rossi: A green approach to chemical building blocks. The case of 3-hydroxypropanoic acid. In: Green Chemistry. 13, 2011, p. 1624, doi : 10.1039 / C1GC15052A .
- ↑ a b Peter Dürre: Technical alcohols and ketones . In: Hermann Sahm, Garabed Antranikian, Klaus-Peter Stahmann & Ralf Takors (eds.): Industrial microbiology . Springer Spectrum, Berlin, Heidelberg 2013, ISBN 978-3-8274-3039-7 , chap. 4 , p. 77-81 , doi : 10.1007 / 978-3-8274-3040-3 .
- ^ A b Carl J. Sullivan, Anja Kuenz & Klaus-Dieter Vorlop: Propanediols . In: Ullmann's Encyclopedia of industrial chemistry . Wiley-VCH, Weinheim 2018, doi : 10.1002 / 14356007.a22_163.pub2 .
- ↑ E. Hala, J. Pick, V. Fried, O. Vilim: Vapor-Liquid Equilibrium. 2nd Edition. Pergamon Press, Oxford 1967.
- ↑ Helmut Sattler & Michael Schweitzer: Fibers, 5. Polyester Fibers . In: Ullmann's Encyclopedia of industrial chemistry . Wiley-VCH, Weinheim 2011, p. 25-27 , doi : 10.1002 / 14356007.o10_o01 .
- ^ EJ Salmi: Investigations on ethereal compounds, I. Mitteil .: To the representation of the acetals and ketals. In: Chem. Ber. 71, 1938, pp. 1803-1808. doi: 10.1002 / cber.19380710905 .
- ↑ R. Leutner: On the hydrolysis rate of cyclic acetals. In: monthly Chem. 60, 1932, pp. 317-352. doi: 10.1007 / BF01538573
- ↑ Patent US2021680 : Preparation of methylene ethers. Applied July 29, 1930 , published November 19, 1935 , applicant: ICI , inventor: Samuel Coffey.
- ↑ a b c Entry on propanediol. In: Römpp Online . Georg Thieme Verlag, accessed on September 20, 2019.
- ↑ Oliver Türk: Material use of renewable raw materials. Springer Vieweg, Wiesbaden, 2014, ISBN 978-3-8348-1763-1 , pp. 417-418.
- ↑ Marcel Kluge, Sacha Pérocheau Arnaud & Tobias Robert: 1,3 ‑ Propanediol and its Application in Bio ‑ Based Polyesters for Resin Applications . In: Chemistry Africa . tape 2 , 2019, p. 215-221 , doi : 10.1007 / s42250-018-0026-4 .