Thiolourethanes
As Thiolourethane (also Thiolurethane ) are referred to the representatives of a chemical substance group. These are the thiolesters of the unstable thiolcarbamic acid (H 2 N – COSH). They are regioisomeric to the thionourethanes , which contain a C = S double bond and no carbonyl group .
The substance groups of thiolo- and thionourethanes are often summarized with the vague designation thiourethanes (synonym: thiocarbamates ).
Manufacturing
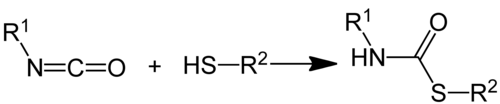
Thiolourethanes can be produced from isocyanates and thioalcohols ( mercaptans ) through an addition reaction :
Properties and application
Thiolourethanes are mostly solids that crystallize well and are used in chemical analysis as identification preparations for thioalcohols. Thioalcohols to be identified are reacted with isocyanates to give thiolourethanes, the melting point of the thiolourethane is measured and compared with tabulated melting points. Today this method has more of a historical significance.
Individual evidence
- ^ Siegfried Hauptmann : Organic Chemistry , VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig, 1985, p. 325. ISBN 3-342-00280-8 .