Aliphatic hydrocarbons

Aliphatic hydrocarbons ( ancient Greek ἄλειφαρ aleiphar 'fatty') are organic chemical compounds that are composed of carbon and hydrogen and are not aromatic . This makes them a subgroup of hydrocarbons . According to the IUPAC nomenclature, aliphatic compounds are “ acyclic or cyclic , saturated or unsaturated carbon compounds, with the exception of aromatic compounds.” The simplest group of aliphatic hydrocarbons are the saturated alkanes ; Furthermore, the likewise saturated cycloalkanes and the unsaturated hydrocarbons of the alkenes and alkynes belong to the group of aliphatics.
Like all pure hydrocarbons, aliphatic hydrocarbons are non-polar, lipophilic compounds (i.e. not water-soluble). Since, according to the IUPAC definition, aliphatic compounds are opposed to aromatic carbon compounds and thus defined by a negation, this means, conversely, that all non- aromatic organic compounds are aliphatic. The classification of organic compounds into aliphatics and aromatics is based on the aromaticity criteria . The so-called alicyclic compounds form a subgroup of the aliphatics and are characterized - similar to the aromatics - by ring-shaped chains, but are distinguished from the aromatics by the aromaticity criteria.
Exemplary aliphatic compounds
The most important groups of substances of aliphatic compounds according to the above definition are:
- n -alkanes, iso- alkanes and cycloalkanes (saturated hydrocarbons),
- straight-chain, branched and cyclic alkenes (unsaturated hydrocarbons) and
- Alkynes (also unsaturated hydrocarbons).
Important examples of aliphatic compounds can be found in the following table (arranged according to the increasing number of carbon or hydrogen atoms):
| formula | Surname | CAS number | Structural formula | Substance group | Synonyms |
|---|---|---|---|---|---|
| CH 4 | methane | 74-82-8 | 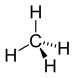 |
Alkane | - |
| C 2 H 2 | Ethine | 74-86-2 | Alkyne | Ethine, acetylene, acetylene | |
| C 2 H 4 | Ethene | 74-85-1 | 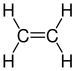 |
Alkene | Ethylene, ethene, ethylene, Elaylgas, vinyl hydrogen, etherin, acetane, R 1150 |
| C 2 H 6 | Ethane | 74-84-0 | 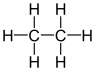 |
Alkane | Ethane |
| C 3 H 4 | Propyne | 74-99-7 |  |
Alkyne | Methyl acetylene, allylene |
| C 3 H 6 | Propene | 115-07-1 | 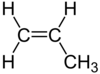 |
Alkene | Propylene |
| C 3 H 8 | propane | 74-98-6 |  |
Alkane | |
| C 4 H 6 | 1,2-butadiene | 590-19-2 |  |
Service | Buta-1,2-diene, methylallene |
| C 4 H 6 | 1-butyne | 107-00-6 |  |
Alkyne | Ethyl acetylene |
| C 4 H 8 | Butene | - | z. B.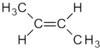
|
Alkene | Butylene |
| C 4 H 10 | n -Butane | 106-97-8 | 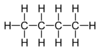 |
Alkane | |
| C 5 H 12 | n -pentane | 109-66-0 | Alkane | Amyl hydride | |
| C 6 H 10 | Cyclohexene | 110-83-8 | Cycloalkene | 1,2,3,4-tetrahydrobenzene | |
| C 7 H 14 | Cycloheptane | 291-64-5 | Cycloalkane | Heptamethylene | |
| C 7 H 14 | Methylcyclohexane | 108-87-2 | 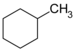 |
Cycloalkane | Hexahydrotoluene, cyclohexyl methane |
| C 8 H 8 | Cuban | 277-10-1 |  |
Pentacyclo [4.2.0.0 2.5 .0 3.8 .0 4.7 ] octane | |
| C 9 H 20 | Nonane | 111-84-2 | Alkane | n -Nonan | |
| C 10 H 12 | Dicyclopentadiene | 77-73-6 |  |
Diene, cycloalkene | 3a, 4,7,7a-Tetrahydro-4,7-methanoindene, tricyclo [5.2.1.0 2,6 ] deca-3,8-diene, TCD, DCPD, dimeric cyclopentadiene |
| C 10 H 16 | Phellandras | 99-83-2 |
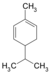 
|
Terpene, diene, cycloalkene | α-Phellandrene: 2-methyl-5- (1-methylethyl) -1,3-cyclohexadiene, β-Phellandrene: 3-methylene-6- (1-methylethyl) cyclohexene |
| C 10 H 16 | α-terpinene | 99-86-5 |  |
Terpene, cycloalkene, diene | Mentha-1,3-diene, 1-isopropyl-4-methyl-1,3-cyclohexadiene |
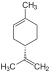
| C 10 H 16 | Limes | 5989-27-5 |
|
Terpene, diene, cycloalkene | 1-methyl-4-prop-1-en-2-ylcyclohexene, Carven, p-mentha-1,8-diene, 1-methyl-4-isopropenyl-1-cyclohexene, 1-methyl-4- (1-methylethenyl ) cyclohexene, 4-isopropenyl-1-methylcylohexene, dipentene, rubber, cinen, cajeputene |
| C 11 H 24 | Undecane | 1120-21-4 | Alkane | Undecan, n -Undecan, n -Undecan, Hendekan | |
| C 30 H 50 | Squalene | 111-02-4 | Terpene, polyene | 2,6,10,15,19,23-hexamethyl- 2,6,10,14,18,22-tetracosahexaene, spinacene, supraen |
|
| C 2n H 4n | Polyethylene | 9002-88-4 |  |
Alkane | Polyethene, PE |
Aliphatic compounds in spectroscopy
Aliphatic CH stretching vibrations of non- conjugated CH units in a molecule have characteristic peaks in the IR spectrum in the range from 3000 to 2750 cm −1 . In contrast, the peaks in conjugated CH units are beyond the 3000 cm −1 limit.
In 1 H- NMR spectrum, most aliphatic peaks in the range of 1-2 ppm are. Higher values up to about 5 ppm are obtained for adjacent electron withdrawing groups.
Aliphatic compounds in petrochemicals
The main source of aliphatic compounds is petroleum. The most important method of oil processing is steam cracking , in which u. a. Ethane , LPG , naphtha and gas oil or other suitable hydrocarbons can be cracked. The gas phase of the steam cracker products contains the basic aliphatic chemicals ethylene , propylene , the C4 cut (mainly butene , isobutene and 1,3-butadiene ) and isoprene .
The most important aliphatic by-products from this are made from the substances eth (yl) en, prop (yl) en and but (yl) en. There are:
- from ethylene :
-
Polyethylene - e.g. B. Ziegler-Natta method
- approx. 21% of the total ethylene production in LDPE
- approx. 13% as LLDPE
- approx. 23% as HDPE
-
α-olefins
- Poly-α-olefins as lubricants
- Co-monomers for polyethylene
-
Polyethylene - e.g. B. Ziegler-Natta method
- made of propylene :
- Polypropylene - e.g. B. by Ziegler-Natta process (approx. 57% of the total propylene production)
- from butene :
- Monomers and co-monomers (butene derived products in plastics production )
- Isobutene - as a monomer for copolymerization with isoprene
- 1,3-butadiene - monomer or co-monomer for polymerisation into elastomers
Web links
- Glossary of Class Names of Organic Compounds and Reactive Intermediates Based on Structure (IUPAC Recommendations 1994): "Acyclic Hydrocarbons" , Department of Chemistry, Queen Mary University of London.
Individual evidence
- ^ Aliphatic compounds. (PDF; 4 kB) 2nd Edition. (No longer available online.) IUPAC Compendium of Chemical Terminology, 1997, archived from the original on November 14, 2013 ; accessed on June 14, 2020 (English).
- ↑ ChemgaPedia steam cracking .