Ethyl chloroformate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
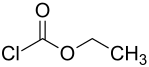
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethyl chloroformate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 5 ClO 2 | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 108.53 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.14 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−81 ° C |
|||||||||||||||
| boiling point |
95 ° C |
|||||||||||||||
| Vapor pressure |
54 h Pa (20 ° C) |
|||||||||||||||
| solubility |
reacts with water |
|||||||||||||||
| Refractive index |
1.3974 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−505.3 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethyl chloroformate is a chemical compound that serves as an intermediate in the production of various carbonic acid derivatives.
Extraction and presentation
Ethyl chloroformate is obtained from phosgene and anhydrous ethanol .
Chemical properties
Chloroformic acid ethyl ester belongs to the group of chloroformic acid esters , as well as chloroformic acid methyl ester , chloroformic acid n- butyl ester , chloroformic acid phenyl ester and chloroformic acid benzyl ester . The connection is thermally unstable. A DSC measurement shows an exothermic decomposition reaction from 136 ° C with an exothermicity of −344 kJ kg −1 or −37.3 kJ mol −1 .
use
Ethyl chloroformate is a widely used intermediate in the production of carbonates and carbamates , which are used in the pharmaceutical industry as starting materials for syntheses.
nomenclature
The common name "ethyl chloroformate" is incorrect, as it is a derivative of carbonic acid , not a derivative of formic acid . It is the monochloride and at the same time the monoethyl ester of carbonic acid.
safety instructions
In concentrations between 3.2 and 27.5 percent by volume, the vapors of ethyl chloroformate form an explosive mixture with air ( flash point 16 ° C, ignition temperature 450 ° C). The compound decomposes when exposed to heat, releasing hydrogen chloride , phosgene, chlorine and other toxic substances.
Individual evidence
- ↑ a b c Data sheet ethyl chloroformate (PDF) from Merck , accessed on February 4, 2018.
- ↑ a b Entry on chloroformic acid ester. In: Römpp Online . Georg Thieme Verlag, accessed on August 29, 2012.
- ↑ a b c data sheet Ethyl chloroformate at AlfaAesar, accessed on February 4, 2018 ( PDF )(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-240.
- ↑ a b Entry on ethyl chloroformate in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Entry on Ethyl chloroformate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ a b chemicalland21.com: Product information .
- ↑ Sperry, JB; Minteer, CJ; Tao, J .; Johnson, R .; Duzguner, R .; Hawksworth, M .; Oke, S .; Richardson, PF; Barnhart, R .; Bill, DR; Giusto, RA; Weaver, JD: Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing in Org. Process Res. Dev. 22 (2018) 1262-1275, doi : 10.1021 / acs.oprd.8b00193 .



