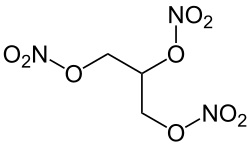
Nitroglycerin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Nitroglycerin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 5 N 3 O 9 | |||||||||||||||
| Brief description |
yellowish, oily liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 227.09 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.59 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
160 ° C (20 hPa) |
|||||||||||||||
| Vapor pressure |
0.5 Pa (30 ° C) |
|||||||||||||||
| solubility |
bad in water |
|||||||||||||||
| Refractive index |
1.4786 (12 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−370.9 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Nitroglycerin (also nitroglycerin ), glycerin trinitrate or glycerol trinitrate , also trisnitric acid glycerol ester is an explosive . It is also used as a medicinal substance with a vasodilating effect. The substance falls within the scope of the Explosives Act and is divided into substance group A in Appendix II.
Surname
The common name nitroglycerin is misleading according to the IUPAC nomenclature, as the prefix nitro indicates a carbon- bound NO 2 group. In glycerol trinitrate, however, the alkyl radical is bound to one nitrogen atom each via a bridging oxygen atom , which is why it is a trisnitric acid ester .
The name glycerol tris (nitrate) would therefore be even more precise than glycerol trinitrate , with the prefix tris indicating that the acid residue is not a condensate of three molecules of HNO 3 , but has been esterified three times with one molecule each. Since the designation of glycerol only the trivial name of the trihydric alcohol propane-1,2,3-triol is (that is, so-called for its Salpetersäureester nitroglycerin ) the designation propanetriol also correct.
history
In 1847 the Turin physician and chemist Ascanio Sobrero produced nitroglycerin for the first time. In 1867 Alfred Nobel invented the safe-to-handle explosive dynamite , in which diatomaceous earth is impregnated with nitroglycerine. In 1875 Nobel manufactured the hitherto strongest commercial explosive, high-explosive gelatine , from nitroglycerine and cellulose nitrate (collodion) . A short time later, this mixture was successfully used in the hard rock during the construction of the Gotthard tunnel in Switzerland. The weaker gelatine dynamites were made from the blasting gelatine with additives . Using ammonium nitrate (ammonium nitrate) in these mixtures, Nobel laid the foundations for the explosives used today.
The English physician William Murrell (1853–1912) prescribed sublingual nitroglycerin in 1879 for the prophylaxis and relief of acute attacks of angina pectoris . In 1924 the pharmacist Kurt Boskamp (1884–1945) formulated the first gelatin capsule with nitroglycerin, Nitrolingual , which is produced by the Pohl-Boskamp company and is still on the market.
Presentation and extraction
Glycerin trinitrate is made by esterifying the three hydroxyl groups of anhydrous glycerin with a mixture of nitric acid and sulfuric acid , called nitrating acid :
A distinction is made between discontinuous and continuous manufacturing processes. In the case of discontinuous processes, a certain amount of nitrating acid is initially charged and, with strong cooling, small amounts of glycerine are added. However, due to the heat development and autocatalytic decomposition at temperatures above 30 ° C, these methods often involve incalculable risks. The occurrence of nitroglycerin vapors can lead to a loss of consciousness because of the blood pressure lowering effect (see above), which makes it impossible to control the temperatures during production and thus makes uncontrolled decomposition likely.
In order to keep the amounts of glycerol trinitrate in the individual processing stages as low as possible and to increase productivity, continuous production processes have therefore been developed. In the simplest case, nitrating acid and glycerine are continuously fed into a cooled pipe system and mix there due to the laminar flow conditions . The most modern methods use injector pumps in which the flowing nitrating acid creates a negative pressure with which the glycerine is sucked in and swirled in the acid jet. The reaction temperature is around 70 ° C.
In general, the synthesis of glycerol trinitrate requires special care and knowledge in handling hazardous substances , so it may only be produced in professional laboratories or technical production facilities. In addition to the obvious dangers of unwanted detonation, which are well known even to most lay people, the toxicological properties of the end product glycerol nitrate (which, among other things, when absorbed through the lungs quickly lead to a massive drop in blood pressure and, in the worst case, to circulatory collapse and Can lead to death), as well as other sources of danger - such as nitrating acid or its highly toxic 'nitrous gases' (nitrogen dioxide) should not be underestimated! Since glycerol nitrate can also be absorbed transdermally (through the skin) and has a lethal effect even in relatively small doses, specially impregnated work clothing is required to protect the extremities in addition to a respirator.
properties
Under standard conditions, glycerol trinitrate is a colorless, odorless and poorly water-soluble liquid. It has a sweet taste, and even taking a small amount of glycerol trinitrate (10 mg or 0.15 mg / kg body weight) leads to headaches. The melting point is 2.8 ° C or 13.5 ° C, depending on the polymorph .
Glycerin trinitrate explodes from a height of one centimeter during a drop hammer test with a 2 kg drop hammer.
- Type: secondary explosive
- Detonation speed: 6700-8500 m / s (7600 m / s (density: 1.599 g / cm³))
- Lead block bulge : 52 cm³ / g
- Impact sensitivity : 0.2 J
- Sensitivity to friction : no reaction up to 350 N pin load
- Limit diameter steel sleeve test : 24 mm
When glycerol trinitrate explodes or disintegrates, from a chemical point of view, an intramolecular redox reaction - with the nitro groups as oxidizing agents and the carbon atoms as reducing agents - results in the reaction products carbon dioxide, water, nitrogen and nitrogen monoxide:
The complete conversion of the liquid explosive in an extremely short time into products that are gaseous at high temperatures leads to a massive expansion in volume, that is, to high explosive power .
use
explosive
Glycerin trinitrate is used as an explosive . However, due to the strong shock and vibration sensitivity, handling is rather difficult. Alfred Nobel succeeded in storing glycerol trinitrate in kieselguhr in 1867. The resulting dynamite was easier to use. But since its 25 percent share of inactive diatomaceous earth reduced the explosive power, Nobel also produced explosive gelatine in 1875 , an ideally disintegrating mixture of nitroglycerine and gun cotton ( cellulose nitrate , ballistite or cordite ). Glycerin trinitrate was later partially replaced by nitroglycol ( ethylene glycol dinitrate or EGDN ) due to its freezing point at 13.5 ° C (stable, rhombic modification) or 2.8 ° C (labile, triclinic form) , which only occurs at −22 ° C freezes. Nitroglycol, however, is quite volatile and therefore not recommended in warm countries with only a small percentage of explosive oil in the total explosive. Glycerin trinitrate, on the other hand, is still an important component of many propellant powder. When added in small amounts, it increases the explosive power of ammonium nitrate explosives .
medicine
Nitroglycerin breaks down in the body, releasing nitric oxide . Nitric oxide has a relaxing (dilating) effect on smooth muscle cells . By acting on the smooth muscle cells in the walls of the blood vessels, it has a vasodilating effect. The effect is particularly strong in large veins and large arteries and less pronounced in resistance vessels . The expansion of the veins reduces the filling pressure of the heart ( preload ), the expansion of the large arteries increases their compliance , the blood pressure falls, and thus the pressure against which the heart has to eject the blood ( afterload ). Both of these lead to the heart having to work less hard and using less oxygen. Therefore, nitroglycerin is used under the name glycerol trinitrate as a drug for ischemic heart diseases such as angina pectoris and for heart failure . See also organic nitrates . Heart attacks, on the other hand, are referred to as "nitro-resistant", since the vasodilatory effect of the released nitrogen monoxide has no therapeutic effect when the coronary arteries are blocked .
It is also used in emergency medicine for left heart failure and cardiac pulmonary edema . Further areas of application are the hypertensive crisis (nitroglycerin, administered intravenously in adults at 25–300 µg / min, lowers both blood pressure and vascular resistance) and spastic ureter and biliary colic . It should be noted, however, that life-threatening complications can occur if the drug Sildenafil ( Viagra ) is taken up to 72 hours before taking the preparation.
An application for a stroke in the form of an emergency plaster is currently being researched.
Side effects
The side effects of the drug include an increase in intracranial pressure , a possible reflex tachycardia, as well as headache, flushing and a feeling of heat.
Trade names
Corangin Nitrospray (D), Deponit (D, A, CH), Glytrin (A), Minitran TM (CH), Minitrans (D), Nitrangin (D), Nitro (D, A), Nitroderm (D, A, CH ), Nitro-Dur (A, CH), Nitronal (CH), Nitrolingual (D, A), Perlinganit (D, A, CH), Trinitrin (CH), Trinitrosan (D), Rectogesic (ointment), various generics ( CH).
Individual evidence
- ↑ a b c d e f Josef Köhler, Rudolf Meyer, Axel Homburg: Explosivstoffe. 10th edition. Wiley-VCH, Weinheim 2008, ISBN 978-3-527-32009-7 .
- ↑ a b c d e f g h i Entry for CAS no. 55-63-0 in the GESTIS substance database of the IFA , accessed on May 10, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-510.
- ↑ Entry on Glycerol trinitrate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ Entry on nitroglycerin in the hazardous substance database in "CLAKS" at the University of Hamburg
- ↑ List of MAK and BAT Values 2013.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 55-63-0 or nitroglycerin ), accessed on November 2, 2015.
- ↑ a b c d Entry on nitroglycerin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Spravochnik po Toksikologii i Gigienicheskim Normativam. P. 180, 1999.
- ↑ a b Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 13, p. 3649, 1985.
- ^ Richard Wuerz, Greg Swope, Steven Meador, C. James Holliman, Gregory S. Roth: Safety of prehospital nitroglycerin . In: Annals of Emergency Medicine . tape 23 , no. 1 , 1994, p. 31-36 , doi : 10.1016 / S0196-0644 (94) 70004-4 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-24.
- ^ Sprengstoffgesetz - SprengG , accessed on November 5, 2018.
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 3-8047-2113-3 , p. 161-162 .
- ^ JR Levick: An Introduction to Cardiovascular Physiology . 5th edition, 2010. Hodder Arnold, London, ISBN 978-0-340-94204-8 . Pages 127, 277, 285.
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. 5th edition. Springer, Berlin / Heidelberg / New York et al. 1999, ISBN 3-540-65024-5 , pp. 65-67. (1st edition 1986). At Google Books
- ↑ Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. 1999, p. 76.
- ^ A b D. Kühn, J. Luxem, K. Runggaldier: Rescue service. 3. Edition. Urban & Fischer, Munich 2004, ISBN 3-437-46191-5 .
- ^ Medicine patch could revolutionize stroke treatment , British Health Foundation, May 5, 2017.
- ↑ Red List online, as of September 2009.
- ↑ AM comp. d. Switzerland, as of September 2009.
- ↑ AGES-PharmMed, as of September 2009.
literature
- Richard Escales: Nitroglycerin & Dynamite . SurvivalPress, 1908. (Reprinted 2002, ISBN 3-8311-4362-5 .)
- Josef Köhler, Rudolf Meyer: Explosives. 9th edition. Wiley-VCH, Weinheim 1998, ISBN 3-527-28864-3 , p. 215 ff.
- Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. 5th edition. Springer, Berlin / Heidelberg / New York et al. 1999, ISBN 3-540-65024-5 , pp. 65-67 and 177 f. (1st edition 1986)
- Alfred Stettbacher: The guns and explosives. 2nd Edition. Leipzig 1933.
Web links

