Iron (III) acetylacetonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
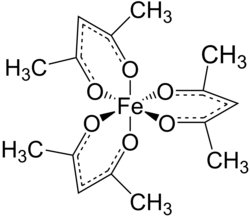
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Iron (III) acetylacetonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | Fe (C 5 H 7 O 2 ) 3 | |||||||||||||||
| Brief description |
dark red solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 353.17 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
5.24 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
180–182 ° C (decomposition) |
|||||||||||||||
| Vapor pressure |
2.6 hPa (110 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Iron (III) acetylacetonate is a chemical compound of iron from the group of acetylacetonates .
Extraction and presentation
Iron (III) acetylacetonate can be produced by reacting iron (III) hydroxide with acetylacetone .
properties
Iron (III) acetylacetonate is a dark red solid that is sparingly soluble in water.
Its red solution turns yellow above 50 ° C and iron (III) hydroxide precipitates when it is boiled.
use
Iron (III) acetylacetonate can be used in Grignard reactions for aryl-alkyl couplings.
The compound can also be used for the production of (Zn, Fe) Fe 2 O 4 layers and measurements of the magnetic properties of these layers, as well as a precursor for the synthesis of water-soluble magnetite nanoparticles, which can be used in the field of magnetic hyperthermia treatment .
It also serves as a catalyst for the curing of polyurethanes in propellants and explosives.
Individual evidence
- ↑ a b c d e f g h i data sheet Iron (III) acetylacetonate, ≥99.9% trace metals basis from Sigma-Aldrich , accessed on March 14, 2019 ( PDF ).
- ↑ a b c data sheet iron (III) acetylacetonate (PDF) from Merck , accessed on March 14, 2019.
- ↑ Entry on iron (III) acetylacetonate. In: Römpp Online . Georg Thieme Verlag, accessed on March 14, 2019.
- ↑ Google Patents: US20040127690A1 - Process for making metal acetylacetonates - Google Patents , accessed March 14, 2019
- ↑ Leopold Gmelin: Iron Part B - Delivery 3: Connections iron and carbon (continued) . Springer-Verlag, 2013, ISBN 978-3-662-13288-3 , p. 555 ( limited preview in Google Book search).
- ↑ A. Fürstner, A. Leitner, G. Seidel: 4-Nonylbenzoic Acid In: Organic Syntheses . 81, 2005, pp. 33-42, doi : 10.15227 / orgsyn.081.0033 ( PDF ).
- ^ Josef Köhler, Rudolf Meyer, Axel Homburg: Explosivstoffe . John Wiley & Sons, 2012, ISBN 3-527-66007-0 ( limited preview in Google Book Search).


