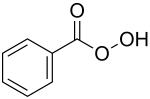
Peroxybenzoic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Peroxybenzoic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 7 H 6 O 3 | |||||||||||||||
| Brief description |
colorless, pungent smelling crystals, very volatile |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 138.12 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
41-43 ° C |
|||||||||||||||
| boiling point |
97–110 ° C (20 h Pa ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
The peroxybenzoic acid (obsolete: perbenzoic acid ) is an organic acid which is a derivative of benzoic acid is. It belongs to the group of peroxycarboxylic acids .
properties
Peroxybenzoic acid is in the form of colorless, pungent-smelling crystals at room temperature and is very volatile . Their melting point is 41–43 ° C. It is only slightly soluble in water, but easily soluble in organic solvents .
use
It is a strong oxidizing agent and therefore has many uses.
With alkenes , peroxybenzoic acid produces epoxides ( Prileschajew reaction ), which is why it is used in analytical chemistry to determine the content of double bonds .
Manufacturing
Peroxybenzoic acid is made from dibenzoyl peroxide and sodium ethoxide in ethanol .
The formation of peroxybenzoic acid during the oxidation of benzaldehyde with hydrogen peroxide was described by Hermann Staudinger as early as 1913.
Individual evidence
- ↑ a b c d e f J. Falbe, M. Regitz (ed.): Römpp Lexikon Chemie . 10th edition, Thieme, Stuttgart a. New York, 1996-1999. P. 3205.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ H. Staudinger: About the auto-oxidation of organic compounds I. About the auto-oxidation of aromatic aldehydes . In: Reports of the German Chemical Society . tape 46 , no. 3 , July 1913, p. 3530-3535 , doi : 10.1002 / cber.191304603134 ( PDF ).