Peterson olefination
The Peterson olefination (also Peterson reaction, after Donald John Peterson) is a name reaction from organic chemistry. An α-silylated carbanion is eliminated with a ketone (or aldehyde ) to form an alkene as the reaction product. The course of the reaction can proceed differently, depending on whether the reaction is acidic or basic.
Reaction mechanism
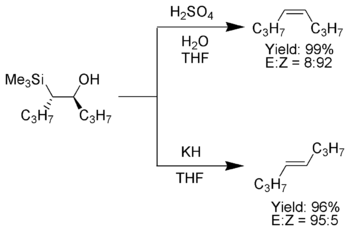
The Peterson olefination is an important reaction in synthetic organic chemistry, as it can be used to diastereoselectively synthesize cis or trans alkenes from β-hydroxysilanes. Working up the β-hydroxysilane with acid gives one alkene, while working up the same β-hydroxysilane with a base gives the alkene with opposite stereochemistry.
- Acid Elimination (top):
The treatment of the β-hydroxysilane with acid results in anti- elimination via protonation .
- Basic elimination (below):
The influence of the base on the β-hydroxysilane leads to a concerted syn elimination.
Since two different stereoisomers can arise in the first step, there are a total of four possible reaction paths:
Alkyl substituents
If the α-silyl carbanion only carries alkyl , hydrogen , or electron donating substituents, the stereochemistry of the Peterson olefination can be controlled, since at a sufficiently low temperature the elimination proceeds so slowly that the intermediate β-hydroxysilane can be isolated.
Once isolated, the diastereomeric β-hydroxysilanes can be separated. One diastereomer is treated with acid while the other is treated with base, resulting in the diastereoselective generation of cis and trans alkene.
Electron withdrawing substituents
If the α-silylated carbanion carries electron-withdrawing substituents, the β-hydroxysilane intermediate is too unstable to be isolated, and the alkene is formed immediately.
literature
Review article:
- Birkofer, L .; Stiehl, O. Top. Curr. Chem. 1980 , 88 , 58.
- Ager, DJ Synthesis 1984 , 384-398.
- Ager, DJ Org.React. 1990 , 38 , 1-223.
- Chan, T.-H. Acc. Chem. Res. 1977 , 10 , 442.
- László Kürti & Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis , 2005, Elsevier Academic Press, pp. 344-345, ISBN 978-0-12-369483-6 .
See also
Individual evidence
- ↑ Peterson, DJ J. Org. Chem. 1968 , 33 , 780.
- ↑ Anthony GM Barrett, John A. Flygare, Jason M. Hill, and Eli M. Wallace: Stereoselective Alkene Synthesis via 1-Chloro-1 - ((Dimethyl) Phenylsilyl) Alkanes AND α- (Dimethyl) Phenylsilyl Ketones: 6-Methyl -6-Dodecene In: Organic Syntheses . 73, 1996, p. 50, doi : 10.15227 / orgsyn.073.0050 ; Coll. Vol. 9, 1998, p. 580 ( PDF ).
- ↑ Ager, DJ Org.React. 1990 , 38 , 1-223.